GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features
- PMID: 24497534
- PMCID: PMC4014843
- DOI: 10.1182/blood-2013-12-544809
GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features
Abstract
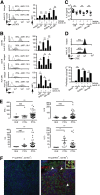
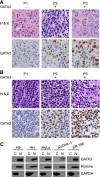
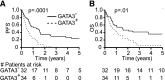
The cell of origin and the tumor microenvironment's role remain elusive for the most common peripheral T-cell lymphomas (PTCLs). As macrophages promote the growth and survival of malignant T cells and are abundant constituents of the tumor microenvironment, their functional polarization was examined in T-cell lymphoproliferative disorders. Cytokines that are abundant within the tumor microenvironment, particularly interleukin (IL)-10, were observed to promote alternative macrophage polarization. Macrophage polarization was signal transducer and activator of transcription 3 dependent and was impaired by the Janus kinase inhibitor ruxolitinib. In conventional T cells, the production of T helper (Th)2-associated cytokines and IL-10, both of which promote alternative macrophage polarization, is regulated by the T-cell transcription factor GATA-binding protein 3 (GATA-3). Therefore, its role in the T-cell lymphomas was examined. GATA-3 expression was observed in 45% of PTCLs, not otherwise specified (PTCL, NOS) and was associated with distinct molecular features, including the production of Th2-associated cytokines. In addition, GATA-3 expression identified a subset of PTCL, NOS with distinct clinical features, including inferior progression-free and overall survival. Collectively, these data suggest that further understanding the cell of origin and lymphocyte ontogeny among the T-cell lymphomas may improve our understanding of the tumor microenvironment's pathogenic role in these aggressive lymphomas.
Figures


Comment in
-
Cellular origin of T-cell lymphomas.Blood. 2014 May 8;123(19):2909-10. doi: 10.1182/blood-2014-02-555763. Blood. 2014. PMID: 24810625 No abstract available.
References
-
- Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159–2169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

