European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma
- PMID: 24497560
- PMCID: PMC3912952
- DOI: 10.3324/haematol.2013.099358
European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma
Abstract
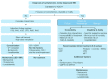
Multiple myeloma management has undergone profound changes in the past thanks to advances in our understanding of the disease biology and improvements in treatment and supportive care approaches. This article presents recommendations of the European Myeloma Network for newly diagnosed patients based on the GRADE system for level of evidence. All patients with symptomatic disease should undergo risk stratification to classify patients for International Staging System stage (level of evidence: 1A) and for cytogenetically defined high- versus standard-risk groups (2B). Novel-agent-based induction and up-front autologous stem cell transplantation in medically fit patients remains the standard of care (1A). Induction therapy should include a triple combination of bortezomib, with either adriamycin or thalidomide and dexamethasone (1A), or with cyclophosphamide and dexamethasone (2B). Currently, allogeneic stem cell transplantation may be considered for young patients with high-risk disease and preferably in the context of a clinical trial (2B). Thalidomide (1B) or lenalidomide (1A) maintenance increases progression-free survival and possibly overall survival (2B). Bortezomib-based regimens are a valuable consolidation option, especially for patients who failed excellent response after autologous stem cell transplantation (2A). Bortezomib-melphalan-prednisone or melphalan-prednisone-thalidomide are the standards of care for transplant-ineligible patients (1A). Melphalan-prednisone-lenalidomide with lenalidomide maintenance increases progression-free survival, but overall survival data are needed. New data from the phase III study (MM-020/IFM 07-01) of lenalidomide-low-dose dexamethasone reached its primary end point of a statistically significant improvement in progression-free survival as compared to melphalan-prednisone-thalidomide and provides further evidence for the efficacy of lenalidomide-low-dose dexamethasone in transplant-ineligible patients (2B).
Figures

References
-
- Engelhardt M, Kleber M, Udi J, Wäsch R, Spencer A, Patriarca F, et al. Consensus statement from European experts on the diagnosis, management, and treatment of multiple myeloma: from standard therapy to novel approaches. Leuk Lymphoma. 2010;51(8): 1424–43 - PubMed
-
- Kleber M, Udi J, Metzke B, Terpos E, Roodmann GD, Morgan G, et al. Challenging the current approaches to multiple myeloma- and other cancer-related bone diseases: from bisphosphonates to targeted therapy. Leuk Lymphoma. 2012;53(6): 1057–61 - PubMed
-
- Kortuem KM, Engelhardt M, Rasche L, Knop S, Einsele H. [Multiple myeloma]. Internist (Berl). 2013;54(8):963–77 - PubMed
-
- Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121(6):893–7 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

