The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure
- PMID: 24497580
- PMCID: PMC3986843
- DOI: 10.1096/fj.13-239509
The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure
Erratum in
-
Erratum. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure.FASEB J. 2015 Jun;29(6):2679. doi: 10.1096/fj.13-239509ERR. FASEB J. 2015. PMID: 26032479 Free PMC article. No abstract available.
Abstract
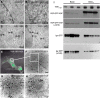
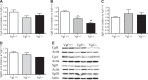
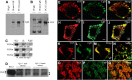
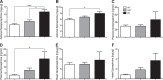
Secretion of proteins and neurotransmitters from large dense core vesicles (LDCVs) is a highly regulated process. Adrenal LDCV formation involves the granin proteins chromogranin A (CgA) and chromogranin B (CgB); CgA- and CgB-derived peptides regulate catecholamine levels and blood pressure. We investigated function of the granin VGF (nonacronymic) in LDCV formation and the regulation of catecholamine levels and blood pressure. Expression of exogenous VGF in nonendocrine NIH 3T3 fibroblasts resulted in the formation of LDCV-like structures and depolarization-induced VGF secretion. Analysis of germline VGF-knockout mouse adrenal medulla revealed decreased LDCV size in noradrenergic chromaffin cells, increased adrenal norepinephrine and epinephrine content and circulating plasma epinephrine, and decreased adrenal CgB. These neurochemical changes in VGF-knockout mice were associated with hypertension. Germline knock-in of human VGF1-615 into the mouse Vgf locus rescued the hypertensive knockout phenotype, while knock-in of a truncated human VGF1-524 that lacks several C-terminal peptides, including TLQP-21, resulted in a small but significant increase in systolic blood pressure compared to hVGF1-615 mice. Finally, acute and chronic administration of the VGF-derived peptide TLQP-21 to rodents decreased blood pressure. Our studies establish a role for VGF in adrenal LDCV formation and the regulation of catecholamine levels and blood pressure.
Keywords: CG; LDCV; adrenal; chromaffin granule; large dense core vesicle; norepinephrine.
Figures


Similar articles
-
VGF and Its C-Terminal Peptide TLQP-62 Regulate Memory Formation in Hippocampus via a BDNF-TrkB-Dependent Mechanism.J Neurosci. 2015 Jul 15;35(28):10343-56. doi: 10.1523/JNEUROSCI.0584-15.2015. J Neurosci. 2015. PMID: 26180209 Free PMC article.
-
Impaired maturation of large dense-core vesicles in muted-deficient adrenal chromaffin cells.J Cell Sci. 2015 Apr 1;128(7):1365-74. doi: 10.1242/jcs.161414. Epub 2015 Feb 11. J Cell Sci. 2015. PMID: 25673877
-
Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles.J Neurosci. 2010 Jan 20;30(3):950-7. doi: 10.1523/JNEUROSCI.2894-09.2010. J Neurosci. 2010. PMID: 20089903 Free PMC article.
-
Granins and catecholamines: functional interaction in chromaffin cells and adipose tissue.Adv Pharmacol. 2013;68:93-113. doi: 10.1016/B978-0-12-411512-5.00005-1. Adv Pharmacol. 2013. PMID: 24054141 Review.
-
Chromogranins A and B as regulators of vesicle cargo and exocytosis.Cell Mol Neurobiol. 2010 Nov;30(8):1181-7. doi: 10.1007/s10571-010-9584-y. Epub 2010 Nov 3. Cell Mol Neurobiol. 2010. PMID: 21046455 Free PMC article. Review.
Cited by
-
VGF and Its C-Terminal Peptide TLQP-62 Regulate Memory Formation in Hippocampus via a BDNF-TrkB-Dependent Mechanism.J Neurosci. 2015 Jul 15;35(28):10343-56. doi: 10.1523/JNEUROSCI.0584-15.2015. J Neurosci. 2015. PMID: 26180209 Free PMC article.
-
Role of a VGF/BDNF/TrkB Autoregulatory Feedback Loop in Rapid-Acting Antidepressant Efficacy.J Mol Neurosci. 2019 Jul;68(3):504-509. doi: 10.1007/s12031-018-1124-0. Epub 2018 Jul 18. J Mol Neurosci. 2019. PMID: 30022437 Free PMC article. Review.
-
Neuroendocrine Role for VGF.Front Endocrinol (Lausanne). 2015 Feb 2;6:3. doi: 10.3389/fendo.2015.00003. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25699015 Free PMC article. Review.
-
VGF Peptides in Cerebrospinal Fluid of Patients with Dementia with Lewy Bodies.Int J Mol Sci. 2019 Sep 20;20(19):4674. doi: 10.3390/ijms20194674. Int J Mol Sci. 2019. PMID: 31547145 Free PMC article. Clinical Trial.
-
Identification of VGF nerve growth factor inducible-producing cells in human spinal cords and expression change in patients with amyotrophic lateral sclerosis.Int J Med Sci. 2020 Feb 4;17(4):480-489. doi: 10.7150/ijms.39101. eCollection 2020. Int J Med Sci. 2020. PMID: 32174778 Free PMC article.
References
-
- Huttner W. B., Gerdes H. H., Rosa P. (1991) The granin (chromogranin/secretogranin) family. Trends Biochem. Sci. 16, 27–30 - PubMed
-
- Loh Y. P., Kim T., Rodriguez Y. M., Cawley N. X. (2004) Secretory granule biogenesis and neuropeptide sorting to the regulated secretory pathway in neuroendocrine cells. J. Mol. Neurosci. 22, 63–71 - PubMed
-
- Ozawa H., Takata K. (1995) The granin family–its role in sorting and secretory granule formation. Cell Struct. Funct. 20, 415–420 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

