Breast metastases from extramammary malignancies: typical and atypical ultrasound features
- PMID: 24497788
- PMCID: PMC3909857
- DOI: 10.3348/kjr.2014.15.1.20
Breast metastases from extramammary malignancies: typical and atypical ultrasound features
Abstract
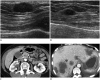
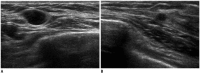
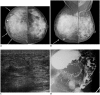
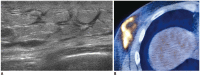
Breast metastases from extramammary malignancies are uncommon. The most common sources are lymphomas/leukemias and melanomas. Some of the less common sources include carcinomas of the lung, ovary, and stomach, and infrequently, carcinoid tumors, hypernephromas, carcinomas of the liver, tonsil, pleura, pancreas, cervix, perineum, endometrium and bladder. Breast metastases from extramammary malignancies have both hematogenous and lymphatic routes. According to their routes, there are common radiological features of metastatic diseases of the breast, but the features are not specific for metastases. Typical ultrasound (US) features of hematogenous metastases include single or multiple, round to oval shaped, well-circumscribed hypoechoic masses without spiculations, calcifications, or architectural distortion; these masses are commonly located superficially in subcutaneous tissue or immediately adjacent to the breast parenchyma that is relatively rich in blood supply. Typical US features of lymphatic breast metastases include diffusely and heterogeneously increased echogenicities in subcutaneous fat and glandular tissue and a thick trabecular pattern with secondary skin thickening, lymphedema, and lymph node enlargement. However, lesions show variable US features in some cases, and differentiation of these lesions from primary breast cancer or from benign lesions is difficult. In this review, we demonstrate various US appearances of breast metastases from extramammary malignancies as typical and atypical features, based on the results of US and other imaging studies performed at our institution. Awareness of the typical and atypical imaging features of these lesions may be helpful to diagnose metastatic lesions of the breast.
Keywords: Breast; Extramammary; Metastasis; Ultrasound.
Figures
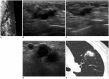
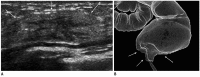
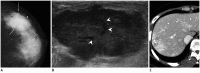
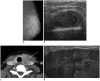

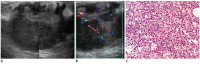
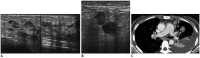
References
-
- Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257–262. - PubMed
-
- McCrea ES, Johnston C, Haney PJ. Metastases to the breast. AJR Am J Roentgenol. 1983;141:685–690. - PubMed
-
- Muttarak M, Nimmonrat A, Chaiwun B. Metastatic carcinoma to the male and female breast. Australas Radiol. 1998;42:16–19. - PubMed
-
- Bohman LG, Bassett LW, Gold RH, Voet R. Breast metastases from extramammary malignancies. Radiology. 1982;144:309–312. - PubMed
-
- Silverman JF, Feldman PS, Covell JL, Frable WJ. Fine needle aspiration cytology of neoplasms metastatic to the breast. Acta Cytol. 1987;31:291–300. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

