CXCR7 is highly expressed in acute lymphoblastic leukemia and potentiates CXCR4 response to CXCL12
- PMID: 24497931
- PMCID: PMC3908922
- DOI: 10.1371/journal.pone.0085926
CXCR7 is highly expressed in acute lymphoblastic leukemia and potentiates CXCR4 response to CXCL12
Erratum in
- PLoS One. 2014;9(3):e91365
Abstract
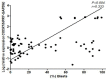
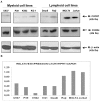
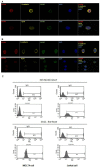
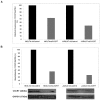
Recently, a novel CXCL12-binding receptor, has been identified. This CXCL12-binding receptor commonly known as CXCR7 (CXC chemokine receptor 7), has lately, based on a novel nomenclature, has received the name ACKR3 (atypical chemokine receptor 3). In this study, we aimed to investigate the expression of CXCR7 in leukemic cells, as well as its participation in CXCL12 response. Interesting, we clearly demonstrated that CXCR7 is highly expressed in acute lymphoid leukemic cells compared with myeloid or normal hematopoietic cells and that CXCR7 contributed to T-acute lymphoid leukemic cell migration induced by CXCL12. Moreover, we showed that the cellular location of CXCR7 varied among T-lymphoid cells and this finding may be related to their migration capacity. Finally, we hypothesized that CXCR7 potentiates CXCR4 response and may contribute to the maintenance of leukemia by initiating cell recruitment to bone marrow niches that were once occupied by normal hematopoietic stem cells.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

