Preclinical evaluation of engineered oncolytic herpes simplex virus for the treatment of pediatric solid tumors
- PMID: 24497984
- PMCID: PMC3907427
- DOI: 10.1371/journal.pone.0086843
Preclinical evaluation of engineered oncolytic herpes simplex virus for the treatment of pediatric solid tumors
Abstract
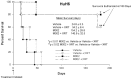
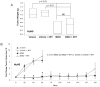
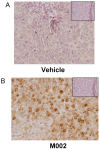
Recently, investigators showed that mice with syngeneic murine gliomas that were treated with a neuroattenuated oncolytic herpes simplex virus-1 (oHSV), M002, had a significant increase in survival. M002 has deletions in both copies of the γ134.5 gene, enabling replication in tumor cells but precluding infection of normal cells. Previous studies have shown antitumor effects of other oHSV against a number of adult tumors including hepatocellular carcinoma and renal cell carcinoma. The purpose of the current study was to investigate the oncolytic potential of M002 against difficult to treat pediatric liver and kidney tumors. We showed that the oHSV, M002, infected, replicated, and decreased cell survival in hepatoblastoma, malignant rhabdoid kidney tumor, and renal sarcoma cell lines. In addition, we showed that in murine xenografts, treatment with M002 significantly increased survival and decreased tumor growth. Finally, these studies showed that the primary entry protein for oHSV, CD111 (nectin-1) was present in human hepatoblastoma and malignant rhabdoid kidney tumor specimens. We concluded that M002 effectively targeted these rare aggressive tumor types and that M002 may have potential for use in children with unresponsive or relapsed pediatric solid tumors.
Conflict of interest statement
Figures





References
-
- Stocker JT (1994) Hepatoblastoma. Semin Diagn Pathol 11: 136–143. - PubMed
-
- Giacomantonio M, Ein SH, Mancer K, Stephens CA (1984) Thirty years of experience with pediatric primary malignant liver tumors. J Pediatr Surg 19: 523–526. - PubMed
-
- Amar AM, Tomlinson G, Green DM, Breslow NE, de Alarcon PA (2001) Clinical presentation of rhabdoid tumors of the kidney. J Pediatr Hematol Oncol 23: 105–108. - PubMed
-
- Zhuge Y, Cheung MC, Yang R, Perez EA, Koniaris LG, et al. (2010) Pediatric non-Wilms renal tumors: subtypes, survival, and prognostic indicators. J Surg Res 163: 257–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

