Pharmacogenetic-based efavirenz dose modification: suggestions for an African population and the different CYP2B6 genotypes
- PMID: 24497997
- PMCID: PMC3909010
- DOI: 10.1371/journal.pone.0086919
Pharmacogenetic-based efavirenz dose modification: suggestions for an African population and the different CYP2B6 genotypes
Abstract
Background: Pharmacogenetics contributes to inter-individual variability in pharmacokinetics (PK) of efavirenz (EFV), leading to variations in both efficacy and toxicity. The purpose of this study was to assess the effect of genetic factors on EFV pharmacokinetics, treatment outcomes and genotype based EFV dose recommendations for adult HIV-1 infected Ugandans.
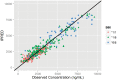
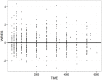
Methods: In total, 556 steady-state plasma EFV concentrations from 99 HIV infected patients (64 female) treated with EFV/lamivudine/zidovidine were analyzed. Patient genotypes for CYP2B6 (*6 & *11), CYP3A5 (*3,*6 & *7) and ABCB1 c.4046A>G, baseline biochemistries and CD4 and viral load change from baseline were determined. A one-compartment population PK model with first-order absorption (NONMEM) was used to estimate genotype effects on EFV pharmacokinetics. PK simulations were performed based upon population genotype frequencies. Predicted AUCs were compared between the product label and simulations for doses of 300 mg, 450 mg, and 600 mg.
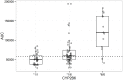
Results: EFV apparent clearance (CL/F) was 2.2 and 1.74 fold higher in CYP2B6*6 (*1/*1) and CYP2B6*6 (*1/*6) compared CYP2B6*6 (*6/*6) carriers, while a 22% increase in F1 was observed for carriers of ABCB1 c.4046A>G variant allele. Higher mean AUC was attained in CYP2B6 *6/*6 genotypes compared to CYP2B6 *1/*1 (p<0.0001). Simulation based AUCs for 600 mg doses were 1.25 and 2.10 times the product label mean AUC for the Ugandan population in general and CYP2B6*6/*6 genotypes respectively. Simulated exposures for EFV daily doses of 300 mg and 450 mg are comparable to the product label. Viral load fell precipitously on treatment, with only six patients having HIV RNA >40 copies/mL after 84 days of treatment. No trend with exposure was noted for these six patients.
Conclusion: Results of this study suggest that daily doses of 450 mg and 300 mg might meet the EFV treatment needs of HIV-1 infected Ugandans in general and individuals homozygous for CYP2B6*6 mutation, respectively.
Conflict of interest statement
Figures
Similar articles
-
Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmacokinetics and treatment outcomes: a parallel-group prospective cohort study in two sub-Saharan Africa populations.PLoS One. 2013 Jul 5;8(7):e67946. doi: 10.1371/journal.pone.0067946. Print 2013. PLoS One. 2013. PMID: 23861838 Free PMC article. Clinical Trial.
-
Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study.J Infect Dis. 2010 Sep 1;202(5):717-22. doi: 10.1086/655470. J Infect Dis. 2010. PMID: 20662624 Free PMC article. Clinical Trial.
-
CYP2B6 polymorphism and nonnucleoside reverse transcriptase inhibitor plasma concentrations in Chinese HIV-infected patients.Ther Drug Monit. 2010 Oct;32(5):573-8. doi: 10.1097/FTD.0b013e3181ea953c. Ther Drug Monit. 2010. PMID: 20625352
-
Efavirenz in the therapy of HIV infection.Expert Opin Drug Metab Toxicol. 2010 Jan;6(1):95-103. doi: 10.1517/17425250903483207. Expert Opin Drug Metab Toxicol. 2010. PMID: 20001610 Free PMC article. Review.
-
Efavirenz Metabolism: Influence of Polymorphic CYP2B6 Variants and Stereochemistry.Drug Metab Dispos. 2019 Oct;47(10):1195-1205. doi: 10.1124/dmd.119.086348. Epub 2019 Jul 19. Drug Metab Dispos. 2019. PMID: 31324697 Free PMC article. Review.
Cited by
-
Predictors of Efavirenz Plasma Exposure, Auto-Induction Profile, and Effect of Pharmacogenetic Variations among HIV-Infected Children in Ethiopia: A Prospective Cohort Study.J Pers Med. 2021 Dec 5;11(12):1303. doi: 10.3390/jpm11121303. J Pers Med. 2021. PMID: 34945777 Free PMC article.
-
Decision-making and role preferences for receiving individual pharmacogenomic research results among participants at a Ugandan HIV research institute.BMC Med Ethics. 2025 Feb 8;26(1):23. doi: 10.1186/s12910-025-01181-w. BMC Med Ethics. 2025. PMID: 39923018 Free PMC article.
-
Incidence of neuropsychiatric side effects of efavirenz in HIV-positive treatment-naïve patients in public-sector clinics in the Eastern Cape.South Afr J HIV Med. 2016 Jun 30;17(1):452. doi: 10.4102/sajhivmed.v17i1.452. eCollection 2016. South Afr J HIV Med. 2016. PMID: 29568611 Free PMC article.
-
The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future.Br J Clin Pharmacol. 2015 Feb;79(2):182-94. doi: 10.1111/bcp.12403. Br J Clin Pharmacol. 2015. PMID: 24730660 Free PMC article. Review.
-
Variability of efavirenz plasma concentrations among pediatric HIV patients treated with efavirenz based combination antiretroviral therapy in Dar es Salaam, Tanzania.BMC Pharmacol Toxicol. 2018 Oct 23;19(1):66. doi: 10.1186/s40360-018-0258-6. BMC Pharmacol Toxicol. 2018. PMID: 30352627 Free PMC article. Clinical Trial.
References
-
- (2009) WHO/HTM/TB/2009.420, Treatment of tuberculosis: guidelines - 4th ed., http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf.2009, WHO Library Cataloguing-in-Publication Data.
-
- Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, et al. (2001) Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15: 71–75. - PubMed
-
- Solas C, Gagnieu MC (2011) [Evidence-based therapeutic drug monitoring for efavirenz]. Therapie 66: 197–205. - PubMed
-
- Burger D, van der Heiden I, la Porte C, van der Ende M, Groeneveld P, et al. (2006) Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol 61: 148–154. - PMC - PubMed
-
- Stohr W, Back D, Dunn D, Sabin C, Winston A, et al. (2008) Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther 13: 675–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

