Peste des petits ruminants virus tissue tropism and pathogenesis in sheep and goats following experimental infection
- PMID: 24498032
- PMCID: PMC3907444
- DOI: 10.1371/journal.pone.0087145
Peste des petits ruminants virus tissue tropism and pathogenesis in sheep and goats following experimental infection
Abstract
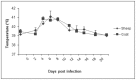
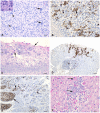
Peste des petits ruminants (PPR) is a viral disease which primarily affects small ruminants, causing significant economic losses for the livestock industry in developing countries. It is endemic in Saharan and sub-Saharan Africa, the Middle East and the Indian sub-continent. The primary hosts for peste des petits ruminants virus (PPRV) are goats and sheep; however recent models studying the pathology, disease progression and viremia of PPRV have focused primarily on goat models. This study evaluates the tissue tropism and pathogenesis of PPR following experimental infection of sheep and goats using a quantitative time-course study. Upon infection with a virulent strain of PPRV, both sheep and goats developed clinical signs and lesions typical of PPR, although sheep displayed milder clinical disease compared to goats. Tissue tropism of PPRV was evaluated by real-time RT-PCR and immunohistochemistry. Lymph nodes, lymphoid tissue and digestive tract organs were the predominant sites of virus replication. The results presented in this study provide models for the comparative evaluation of PPRV pathogenesis and tissue tropism in both sheep and goats. These models are suitable for the establishment of experimental parameters necessary for the evaluation of vaccines, as well as further studies into PPRV-host interactions.
Conflict of interest statement
Figures




References
-
- Gargadennec L, Lalanne A (1942) La peste des petits ruminants. Bulletin des Services Zoo Techniques et des Epizzoties de l’Afrique Occidentale Francaise 5: 16–21.
-
- African Union Internation Bureau for Animal Resources. (2012) Impact of livestock diseases in Africa. : 1–2–7.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

