Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe
- PMID: 24498036
- PMCID: PMC3909048
- DOI: 10.1371/journal.pone.0087160
Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe
Abstract
Background: The impact of immunosuppression despite virological suppression (immuno-virological discordance, ID) on the risk of developing fatal and non-fatal AIDS/non-AIDS events is unclear and remains to be elucidated.
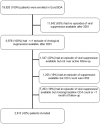
Methods: Patients in EuroSIDA starting at least 1 new antiretroviral drug with CD4<350 cells/µl and viral load (VL)>500 copies/mL were followed-up from the first day of VL< = 50 copies/ml until a new fatal/non-fatal non-AIDS/AIDS event. Considered non-AIDS events included non-AIDS malignancies, pancreatitis, severe liver disease with hepatic encephalopathy (>grade 3), cardio- and cerebrovascular events, and end-stage renal disease. Patients were classified over time according to whether current CD4 count was above (non-ID) or below (ID) baseline level. Relative rates (RR) of events were calculated for ID vs. non-ID using adjusted Poisson regression models.
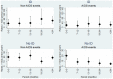
Results: 2,913 patients contributed 11,491 person-years for the analysis of non-AIDS. 241 pre-specified non-AIDS events (including 84 deaths) and 89 AIDS events (including 10 deaths) occurred. The RR of developing pre-specified non-AIDS events for ID vs. non-ID was 1.96 (95% CI 1.37-2.81, p<0.001) in unadjusted analysis and 1.43 (0.94-2.17, p = 0.095) after controlling for current CD4 count. ID was not associated with the risk of AIDS events (aRR 0.76, 95% CI 0.41-1.38, p = 0.361).
Conclusion: Compared to CD4 responders, patients with immuno-virological discordance may be at increased risk of developing non-AIDS events. Further studies are warranted to establish whether in patients with ID, strategies to directly modify CD4 count response may be needed besides the use of ART.
Conflict of interest statement
Figures
References
-
- Hughes MD, Daniels MJ, Fischl MA, Kim S (1998) Schooley RT (1998) CD4 cell count as a surrogate endpoint in HIV clinical trials: a meta-analysis of studies of the AIDS Clinical Trials Group. AIDS 12: 1823–1832. - PubMed
-
- Staszewski S, Miller V, Sabin C, Schlecht C, Gute P, et al. (1999) Determinants of sustainable CD4 lymphocyte count increases in response to antiretroviral therapy. AIDS 13: 951–956. - PubMed
-
- Wolbers M, Bucher HC, Furrer H, Rickenbach M, Cavassini M, et al. (2008) Delayed diagnosis of HIV infection and late initiation of antiretroviral therapy in the Swiss HIV Cohort Study. HIV Med 9: 397–405. - PubMed
-
- Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, et al. (2007) Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet 370: 407–413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials