Fluphenazine reduces proteotoxicity in C. elegans and mammalian models of alpha-1-antitrypsin deficiency
- PMID: 24498058
- PMCID: PMC3909079
- DOI: 10.1371/journal.pone.0087260
Fluphenazine reduces proteotoxicity in C. elegans and mammalian models of alpha-1-antitrypsin deficiency
Abstract
The classical form of α1-antitrypsin deficiency (ATD) is associated with hepatic fibrosis and hepatocellular carcinoma. It is caused by the proteotoxic effect of a mutant secretory protein that aberrantly accumulates in the endoplasmic reticulum of liver cells. Recently we developed a model of this deficiency in C. elegans and adapted it for high-content drug screening using an automated, image-based array scanning. Screening of the Library of Pharmacologically Active Compounds identified fluphenazine (Flu) among several other compounds as a drug which reduced intracellular accumulation of mutant α1-antitrypsin Z (ATZ). Because it is representative of the phenothiazine drug class that appears to have autophagy enhancer properties in addition to mood stabilizing activity, and can be relatively easily re-purposed, we further investigated its effects on mutant ATZ. The results indicate that Flu reverses the phenotypic effects of ATZ accumulation in the C. elegans model of ATD at doses which increase the number of autophagosomes in vivo. Furthermore, in nanomolar concentrations, Flu enhances the rate of intracellular degradation of ATZ and reduces the cellular ATZ load in mammalian cell line models. In the PiZ mouse model Flu reduces the accumulation of ATZ in the liver and mediates a decrease in hepatic fibrosis. These results show that Flu can reduce the proteotoxicity of ATZ accumulation in vivo and, because it has been used safely in humans, this drug can be moved rapidly into trials for liver disease due to ATD. The results also provide further validation for drug discovery using C. elegans models that can be adapted to high-content drug screening platforms and used together with mammalian cell line and animal models.
Conflict of interest statement
Figures
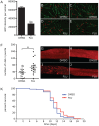
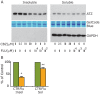
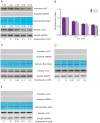

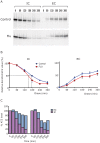
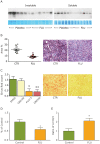
References
-
- Perlmutter DH, Brodsky JL, Balistreri WF, Trapnell BC (2007) Molecular pathogenesis of alpha-1-antitrypsin deficiency-associated liver disease: A meeting review. Hepatology 45: 1313–1323. - PubMed
-
- Perlmutter DH (2010) Alpha-1-antitrypsin deficiency: Importance of proteasomal and autophagic degradative pathways in disposal of liver disease-associated protein aggregates. Annu. Rev. Med. 62: 333–345. - PubMed
-
- Qu D, Teckman JH, Omura S, Perlmutter DH (1996) Degradation of a mutant secretory protein, α1-antitrypsin Z, in the endoplasmic reticulum requires proteasome activity. J. Biol. Chem. 271: 22791–22795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

