Humanized HLA-DR4 mice fed with the protozoan pathogen of oysters Perkinsus marinus (Dermo) do not develop noticeable pathology but elicit systemic immunity
- PMID: 24498105
- PMCID: PMC3909113
- DOI: 10.1371/journal.pone.0087435
Humanized HLA-DR4 mice fed with the protozoan pathogen of oysters Perkinsus marinus (Dermo) do not develop noticeable pathology but elicit systemic immunity
Abstract
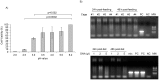
Perkinsus marinus (Phylum Perkinsozoa) is a marine protozoan parasite responsible for "Dermo" disease in oysters, which has caused extensive damage to the shellfish industry and estuarine environment. The infection prevalence has been estimated in some areas to be as high as 100%, often causing death of infected oysters within 1-2 years post-infection. Human consumption of the parasites via infected oysters is thus likely to occur, but to our knowledge the effect of oral consumption of P. marinus has not been investigated in humans or other mammals. To address the question we used humanized mice expressing HLA-DR4 molecules and lacking expression of mouse MHC-class II molecules (DR4.EA(0)) in such a way that CD4 T cell responses are solely restricted by the human HLA-DR4 molecule. The DR4.EA(0) mice did not develop diarrhea or any detectable pathology in the gastrointestinal tract or lungs following single or repeated feedings with live P. marinus parasites. Furthermore, lymphocyte populations in the gut associated lymphoid tissue and spleen were unaltered in the parasite-fed mice ruling out local or systemic inflammation. Notably, naïve DR4.EA(0) mice had antibodies (IgM and IgG) reacting against P. marinus parasites whereas parasite specific T cell responses were undetectable. Feeding with P. marinus boosted the antibody responses and stimulated specific cellular (IFNγ) immunity to the oyster parasite. Our data indicate the ability of P. marinus parasites to induce systemic immunity in DR4.EA(0) mice without causing noticeable pathology, and support rationale grounds for using genetically engineered P. marinus as a new oral vaccine platform to induce systemic immunity against infectious agents.
Conflict of interest statement
Figures



Similar articles
-
Early host-pathogen interactions in marine bivalves: evidence that the alveolate parasite Perkinsus marinus infects through the oyster mantle during rejection of pseudofeces.J Invertebr Pathol. 2013 May;113(1):26-34. doi: 10.1016/j.jip.2012.12.011. Epub 2012 Dec 27. J Invertebr Pathol. 2013. PMID: 23274079
-
First report of the protozoan parasite Perkinsus marinus in South America, infecting mangrove oysters Crassostrea rhizophorae from the Paraíba River (NE, Brazil).J Invertebr Pathol. 2013 May;113(1):96-103. doi: 10.1016/j.jip.2013.02.002. Epub 2013 Feb 22. J Invertebr Pathol. 2013. PMID: 23439264
-
HLA class II (DR0401) molecules induce Foxp3+ regulatory T cell suppression of B cells in Plasmodium yoelii strain 17XNL malaria.Infect Immun. 2014 Jan;82(1):286-97. doi: 10.1128/IAI.00272-13. Epub 2013 Oct 28. Infect Immun. 2014. PMID: 24166949 Free PMC article.
-
The search for the missing link: a relic plastid in Perkinsus?Int J Parasitol. 2011 Oct;41(12):1217-29. doi: 10.1016/j.ijpara.2011.07.008. Epub 2011 Aug 22. Int J Parasitol. 2011. PMID: 21889509 Free PMC article. Review.
-
A review of current state of knowledge concerning Perkinsus marinus effects on Crassostrea virginica (Gmelin) (the eastern oyster).Vet Pathol. 2013 May;50(3):404-11. doi: 10.1177/0300985813480806. Epub 2013 Mar 5. Vet Pathol. 2013. PMID: 23462867 Review.
Cited by
-
Dissemination of Orientia tsutsugamushi, a Causative Agent of Scrub Typhus, and Immunological Responses in the Humanized DRAGA Mouse.Front Immunol. 2018 Apr 30;9:816. doi: 10.3389/fimmu.2018.00816. eCollection 2018. Front Immunol. 2018. PMID: 29760694 Free PMC article.
-
Transient Expression of Plasmodium berghei MSP8 and HAP2 in the Marine Protozoan Parasite Perkinsus marinus.J Parasitol. 2017 Feb;103(1):118-122. doi: 10.1645/16-88. Epub 2016 Oct 10. J Parasitol. 2017. PMID: 27723436 Free PMC article.
-
Perkinsus marinus in bioreactor: growth and a cost-reduced growth medium.J Ind Microbiol Biotechnol. 2023 Feb 17;50(1):kuad023. doi: 10.1093/jimb/kuad023. J Ind Microbiol Biotechnol. 2023. PMID: 37669897 Free PMC article.
-
Protozoan parasites of bivalve molluscs: literature follows culture.PLoS One. 2014 Jun 23;9(6):e100872. doi: 10.1371/journal.pone.0100872. eCollection 2014. PLoS One. 2014. PMID: 24955977 Free PMC article.
-
CRISPR/Cas9 Ribonucleoprotein-Based Genome Editing Methodology in the Marine Protozoan Parasite Perkinsus marinus.Front Bioeng Biotechnol. 2021 Apr 9;9:623278. doi: 10.3389/fbioe.2021.623278. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33898400 Free PMC article.
References
-
- Mackin JG, Owen HM, Collier A (1950) Preliminary note on the occurrence of a new protistan parasite, Dermocystidium marinum n. sp., in Crassostrea virginica (Gmelin). Science 111: 328–329. - PubMed
-
- Mackin JG, Ray SM (1966) The taxonomic relationships of Dermocystidium marinum Mackin, Owen and Collier. J Inverteb Pathol 8: 544–545.
-
- Levine ND (1978) Perkinsus genus and other new taxa in the protozoan phylum Apicomplexa. J Parasitol 64: 549. - PubMed
-
- Goggin CL, Barker SC (1993) Phylogenetic position of the genus Perkinsus (Protista, Apicomplexa) based on small subunit ribosomal RNA. Mol Biochem Parasitol 60: 65–70. - PubMed
-
- Siddall ME, Reece KS, Graves JE, Burreson EM (1997) ‘Total evidence’ refutes the inclusion of Perkinsus species in the phylum Apicomplexa. Parasitology 115: 165–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

