Temporal dynamics of glyoxalase 1 in secondary neuronal injury
- PMID: 24498315
- PMCID: PMC3911945
- DOI: 10.1371/journal.pone.0087364
Temporal dynamics of glyoxalase 1 in secondary neuronal injury
Abstract
Background: Enhanced glycolysis leads to elevated levels of the toxic metabolite methylglyoxal which contributes to loss of protein-function, metabolic imbalance and cell death. Neurons were shown being highly susceptible to methylglyoxal toxicity. Glyoxalase 1 as an ubiquitous enzyme reflects the main detoxifying enzyme of methylglyoxal and underlies changes during aging and neurodegeneration. However, little is known about dynamics of Glyoxalase 1 following neuronal lesions so far.
Methods: To determine a possible involvement of Glyoxalase 1 in acute brain injury, we analysed the temporal dynamics of Glyoxalase 1 distribution and expression by immunohistochemistry and Western Blot analysis. Organotypic hippocampal slice cultures were excitotoxically (N-methyl-D-aspartate, 50 µM for 4 hours) lesioned in vitro (5 minutes to 72 hours). Additionally, permanent middle cerebral artery occlusion was performed (75 minutes to 60 days).
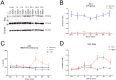
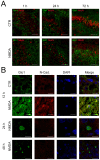
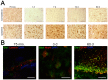
Results: We found (i) a predominant localisation of Glyoxalase 1 in endothelial cells in non-lesioned brains (ii) a time-dependent up-regulation and re-distribution of Glyoxalase 1 in neurons and astrocytes and (iii) a strong increase in Glyoxalase 1 dimers after neuronal injury (24 hours to 72 hours) when compared to monomers of the protein.
Conclusions: The high dynamics of Glyoxalase 1 expression and distribution following neuronal injury may indicate a novel role of Glyoxalase 1.
Conflict of interest statement
Figures
Similar articles
-
Comparative Examination of Temporal Glyoxalase 1 Variations Following Perforant Pathway Transection, Excitotoxicity, and Controlled Cortical Impact Injury.Neurotox Res. 2018 Feb;33(2):412-421. doi: 10.1007/s12640-017-9808-8. Epub 2017 Sep 12. Neurotox Res. 2018. PMID: 28900826
-
Pathological effects of glyoxalase I inhibition in SH-SY5Y neuroblastoma cells.J Neurosci Res. 2006 Jun;83(8):1591-600. doi: 10.1002/jnr.20838. J Neurosci Res. 2006. PMID: 16555297
-
The temporal and spatial dynamics of glyoxalase I following excitoxicity and brain ischaemia.Biochem Soc Trans. 2014 Apr;42(2):534-7. doi: 10.1042/BST20140022. Biochem Soc Trans. 2014. PMID: 24646274 Review.
-
Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons.Int J Biochem Cell Biol. 2008;40(2):245-57. doi: 10.1016/j.biocel.2007.07.019. Epub 2007 Aug 8. Int J Biochem Cell Biol. 2008. PMID: 17869161
-
Glyoxalase pathway of trypanosomatid parasites: a promising chemotherapeutic target.Curr Drug Targets. 2008 Nov;9(11):957-65. doi: 10.2174/138945008786786082. Curr Drug Targets. 2008. PMID: 18991608 Review.
Cited by
-
Ethyl pyruvate does not require microglia for mediating neuroprotection after excitotoxic injury.CNS Neurosci Ther. 2017 Oct;23(10):798-807. doi: 10.1111/cns.12725. Epub 2017 Aug 23. CNS Neurosci Ther. 2017. PMID: 28836378 Free PMC article.
-
On the importance of the innervation of the human cervical longitudinal ligaments at vertebral level.Surg Radiol Anat. 2020 Feb;42(2):127-136. doi: 10.1007/s00276-019-02316-6. Epub 2019 Sep 6. Surg Radiol Anat. 2020. PMID: 31493007
-
Dicarbonyl Stress at the Crossroads of Healthy and Unhealthy Aging.Cells. 2019 Jul 19;8(7):749. doi: 10.3390/cells8070749. Cells. 2019. PMID: 31331077 Free PMC article. Review.
-
Glutathione-Dependent Detoxification Processes in Astrocytes.Neurochem Res. 2015 Dec;40(12):2570-82. doi: 10.1007/s11064-014-1481-1. Epub 2014 Nov 27. Neurochem Res. 2015. PMID: 25428182 Review.
-
Exploratory Application of Neuropharmacometabolomics in Severe Childhood Traumatic Brain Injury.Crit Care Med. 2018 Sep;46(9):1471-1479. doi: 10.1097/CCM.0000000000003203. Crit Care Med. 2018. PMID: 29742587 Free PMC article. Clinical Trial.
References
-
- Thornalley PJ (2003) Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 31 Pt 6: 1343–1348. - PubMed
-
- Phillips SA, Thornalley PJ (1993) The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem 212 1: 101–105. - PubMed
-
- Ahmed N, Battah S, Karachalias N, Babaei-Jadidi R, Horányi M, et al. (2003) Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim Biophys Acta 1639 2: 121–132. - PubMed
-
- Amicarelli F, Colafarina S, Cattani F, Cimini A, Di Ilio C, et al. (2003) Scavenging system efficiency is crucial for cell resistance to ROS-mediated methylglyoxal injury. Free Radic Biol Med 35 8: 856–871. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

