Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab
- PMID: 24498358
- PMCID: PMC3912016
- DOI: 10.1371/journal.pone.0087705
Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab
Abstract
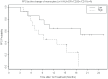
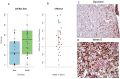
We evaluated neoadjuvant ipilimumab in patients with surgically operable regionally advanced melanoma in order to define markers of activity in the blood and tumor as assessed at baseline (before ipilimumab) and early on-treatment. Patients were treated with ipilimumab (10 mg/kg intravenously every 3 weeks ×2 doses) bracketing surgery. Tumor and blood biospecimens were obtained at baseline and at surgery. Flow cytometry and immunohistochemistry for select biomarkers were performed. Thirty five patients were enrolled; IIIB (3; N2b), IIIC (32; N2c, N3), IV (2). Worst toxicities included Grade 3 diarrhea/colitis (5; 14%), hepatitis (2; 6%), rash (1; 3%), elevated lipase (3; 9%). Median follow up was 18 months: among 33 evaluable patients, median progression free survival (PFS) was 11 months, 95% CI (6.2-19.2). There was a significant decrease in circulating myeloid derived suppressor cells (MDSC). Greater decrease in circulating monocyte gate MDSC Lin1-/HLA-DR-/CD33⁺/CD11b⁺ was associated with improved PFS (p = 0.03). There was a significant increase in circulating regulatory T cells (Treg; CD4⁺CD25hi⁺Foxp3⁺) that, unexpectedly, was associated with improved PFS (HR = 0.57; p = 0.034). Baseline evidence of fully activated type I CD4⁺ and CD8⁺ antigen-specific T cell immunity against cancer-testis (NY-ESO-1) and melanocytic lineage (MART-1, gp100) antigens was detected and was significantly potentiated after ipilimumab. In tumor, there was a significant increase in CD8⁺ T cells after ipilimumab (p = 0.02). Ipilimumab induced increased tumor infiltration by fully activated (CD69⁺) CD3⁺/CD4⁺ and CD3⁺/CD8⁺ T cells with evidence of induction/potentiation of memory T cells (CD45RO⁺). The change in Treg observed within the tumor showed an inverse relationship with clinical benefit and greater decrease in tumor MDSC subset Lin1-/HLA-DR-/CD33⁺/CD11b⁺ was associated with improved PFS at one year. Neoadjuvant evaluation revealed a significant immunomodulating role for ipilimumab on Treg, MDSC and effector T cells in the circulation and tumor microenvironment that warrants further pursuit in the quest for optimizing melanoma immunotherapy.
Conflict of interest statement
Figures


References
-
- Balch CM, Soong SJ, Smith T, Ross MI, Urist MM, et al. (2001) Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann Surg Oncol 8: 101–108. - PubMed
-
- Karakousis CP, Balch CM, Urist MM, Ross MM, Smith TJ, et al. (1996) Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Ann Surg Oncol 3: 446–452. - PubMed
-
- Estevez LG, Gradishar WJ (2004) Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res 10: 3249–3261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

