A randomized clinical trial to evaluate two doses of an intra-articular injection of LMWF-5A in adults with pain due to osteoarthritis of the knee
- PMID: 24498399
- PMCID: PMC3912151
- DOI: 10.1371/journal.pone.0087910
A randomized clinical trial to evaluate two doses of an intra-articular injection of LMWF-5A in adults with pain due to osteoarthritis of the knee
Abstract
Objective: The Low Molecular Weight Fraction of 5% human serum Albumin (LMWF-5A) is being investigated as a treatment for knee pain from osteoarthritis.
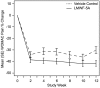
Methods: This was a multicenter randomized, vehicle-controlled, double-blind, parallel study designed to evaluate the safety and efficacy of two doses of an intra-articular injection of LMWF-5A. Patients with symptomatic knee osteoarthritis were randomized 1∶1∶1∶1 to receive a single 4 mL or 10 mL intra-articular knee injection of either LMWF-5A or vehicle control (saline). The primary efficacy endpoint was the difference between treatment groups in the Western Ontario and McMaster Universities (WOMAC) pain change from baseline over 12 weeks. Safety was examined as the incidence and severity of adverse events (AEs).
Results: A total of 329 patients were randomized and received treatment. LMWF-5A resulted in a significant decrease in pain at 12 weeks compared to vehicle control (-0.93 vs -0.72; estimated difference from control: -0.25, p = 0.004); an injection volume effect was not observed (p = 0.64). The effect of LMWF-5A on pain was even more pronounced in patients with severe knee OA (Kellgren Lawrence Grade IV): the estimated difference from control was -0.42 (p = 0.02). Adverse events were generally mild and were similar in patients who received vehicle control (47%) and LMWF-5A (41%).
Conclusions: This clinical trial demonstrated that LMWF-5A is safe and effective at providing relief for the pain of moderate to severe OA of the knee over 12 weeks when administered by intra-articular injection into the knee.
Trial registration: ClinicalTrials.gov NCT01839331.
Conflict of interest statement
Figures
References
-
- Dillon CF, Rasch EK, Gu Q, Hirsch R (2006) Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. The Journal of rheumatology 33: 2271–2279. - PubMed
-
- Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, et al. (2008) Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Archives of internal medicine 168: 1576–1584. - PubMed
-
- American Academy of Orthopaedic Surgeons (2013) Treatment of osteoarthritis of the knee: Evidence-based guideline. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons.
-
- Dunlop DD, Manheim LM, Song J, Chang RW (2001) Arthritis prevalence and activity limitations in older adults. Arthritis and rheumatism 44: 212–221. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical