Human FXR regulates SHP expression through direct binding to an LRH-1 binding site, independent of an IR-1 and LRH-1
- PMID: 24498423
- PMCID: PMC3912179
- DOI: 10.1371/journal.pone.0088011
Human FXR regulates SHP expression through direct binding to an LRH-1 binding site, independent of an IR-1 and LRH-1
Abstract
Background: Farnesoid X receptor/retinoid X receptor-alpha (FXR/RXRα) is the master transcriptional regulator of bile salt synthesis and transport in liver and intestine. FXR is activated by bile acids, RXRα by the vitamin A-derivative 9-cis retinoic acid (9cRA). Remarkably, 9cRA inhibits binding of FXR/RXRα to its response element, an inverted repeat-1 (IR-1). Still, most FXR/RXRα target genes are maximally expressed in the presence of both ligands, including the small heterodimer partner (SHP). Here, we revisited the FXR/RXRα-mediated regulation of human SHP.
Methods: A 579-bp hSHP promoter element was analyzed to locate FXR/chenodeoxycholic acid (CDCA)- and RXRα/9cRA-responsive elements. hSHP promoter constructs were analyzed in FXR/RXRα-transfected DLD-1, HEK293 and HepG2 cells exposed to CDCA, GW4064 (synthetic FXR ligand) and/or 9cRA. FXR-DNA interactions were analyzed by in vitro pull down assays.
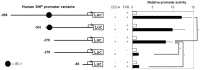
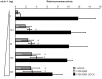
Results: hSHP promoter elements lacking the previously identified IR-1 (-291/-279) largely maintained their activation by FXR/CDCA, but were unresponsive to 9cRA. FXR-mediated activation of the hSHP promoter was primarily dependent on the -122/-69 region. Pull down assays revealed a direct binding of FXR to the -122/-69 sequence, which was abrogated by site-specific mutations in a binding site for the liver receptor homolog-1 (LRH-1) at -78/-70. These mutations strongly impaired the FXR/CDCA-mediated activation, even in the context of a hSHP promoter containing the IR-1. LRH-1 did not increase FXR/RXRα-mediated activation of hSHP promoter activity.
Conclusion: FXR/CDCA-activated expression of SHP is primarily mediated through direct binding to an LRH-1 binding site, which is not modulated by LRH-1 and unresponsive to 9cRA. 9cRA-induced expression of SHP requires the IR-1 that overlaps with a direct repeat-2 (DR-2) and DR-4. This establishes for the first time a co-stimulatory, but independent, action of FXR and RXRα agonists.
Conflict of interest statement
Figures





Similar articles
-
Low retinol levels differentially modulate bile salt-induced expression of human and mouse hepatic bile salt transporters.Hepatology. 2009 Jan;49(1):151-9. doi: 10.1002/hep.22661. Hepatology. 2009. PMID: 19111018
-
Differential regulation of human and mouse orphan nuclear receptor small heterodimer partner promoter by sterol regulatory element binding protein-1.J Biol Chem. 2004 Jul 2;279(27):28122-31. doi: 10.1074/jbc.M313302200. Epub 2004 Apr 30. J Biol Chem. 2004. PMID: 15123650
-
Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor.Mol Endocrinol. 2003 Feb;17(2):259-72. doi: 10.1210/me.2002-0120. Mol Endocrinol. 2003. PMID: 12554753
-
Bile salt excretory pump: biology and pathobiology.J Pediatr Gastroenterol Nutr. 2006 Jul;43 Suppl 1:S10-6. doi: 10.1097/01.mpg.0000226385.71859.5f. J Pediatr Gastroenterol Nutr. 2006. PMID: 16819395 Review.
-
Role of farnesoid X receptor and bile acids in alcoholic liver disease.Acta Pharm Sin B. 2015 Mar;5(2):158-67. doi: 10.1016/j.apsb.2014.12.011. Epub 2015 Mar 9. Acta Pharm Sin B. 2015. PMID: 26579442 Free PMC article. Review.
Cited by
-
Pathogenic mechanisms and regulatory factors involved in alcoholic liver disease.J Transl Med. 2023 May 4;21(1):300. doi: 10.1186/s12967-023-04166-8. J Transl Med. 2023. PMID: 37143126 Free PMC article. Review.
-
The roles of nuclear receptors in cholesterol metabolism and reverse cholesterol transport in nonalcoholic fatty liver disease.Hepatol Commun. 2023 Dec 15;8(1):e0343. doi: 10.1097/HC9.0000000000000343. eCollection 2024 Jan 1. Hepatol Commun. 2023. PMID: 38099854 Free PMC article. Review.
-
The Nuclear Farnesoid X Receptor Reduces p53 Ubiquitination and Inhibits Cervical Cancer Cell Proliferation.Front Cell Dev Biol. 2021 Apr 6;9:583146. doi: 10.3389/fcell.2021.583146. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33889569 Free PMC article.
-
Activation of FXR promotes intestinal metaplasia of gastric cells via SHP-dependent upregulation of the expression of CDX2.Oncol Lett. 2018 May;15(5):7617-7624. doi: 10.3892/ol.2018.8342. Epub 2018 Mar 23. Oncol Lett. 2018. PMID: 29849798 Free PMC article.
-
A practice-changing culture method relying on shaking substantially increases mitochondrial energy metabolism and functionality of human liver cell lines.PLoS One. 2018 Apr 19;13(4):e0193664. doi: 10.1371/journal.pone.0193664. eCollection 2018. PLoS One. 2018. PMID: 29672606 Free PMC article.
References
-
- Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ (2001) Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 276: 28857–28865. - PubMed
-
- Plass JR, Mol O, Heegsma J, Geuken M, Faber KN, et al. (2002) Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology 35: 589–596. - PubMed
-
- Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, et al. (2000) Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6: 507–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

