Leptospira Interrogans induces fibrosis in the mouse kidney through Inos-dependent, TLR- and NLR-independent signaling pathways
- PMID: 24498450
- PMCID: PMC3907306
- DOI: 10.1371/journal.pntd.0002664
Leptospira Interrogans induces fibrosis in the mouse kidney through Inos-dependent, TLR- and NLR-independent signaling pathways
Erratum in
- PLoS Negl Trop Dis. 2014 Apr;8(4):e2802
Abstract
Background: Leptospira (L.) interrogans are bacteria responsible for a worldwide reemerging zoonosis. Rodents carry L. interrogans asymptomatically in their kidneys and excrete bacteria in the urine, contaminating the environment. Humans get infected through skin contact and develop a mild or severe leptospirosis that may lead to renal failure and fibrosis. L. interrogans provoke an interstitial nephritis, but the induction of fibrosis caused by L. interrogans has not been studied in murine models. Innate immune receptors from the TLR and NLR families have recently been shown to play a role in the development and progression of tissue fibrosis in the lung, liver and kidneys under different pathophysiological situations. We recently showed that TLR2, TLR4, and NLRP3 receptors were crucial in the defense against leptospirosis. Moreover, infection of a human cell line with L. interrogans was shown to induce TLR2-dependent production of fibronectin, a component of the extracellular matrix. Therefore, we thought to assess the presence of renal fibrosis in L. interrogans infected mice and to analyze the contribution of some innate immune pathways in this process.
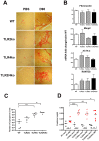
Methodology/principal findings: Here, we characterized by immunohistochemical studies and quantitative real-time PCR, a model of Leptospira-infected C57BL/6J mice, with chronic carriage of L. interrogans inducing mild renal fibrosis. Using various strains of transgenic mice, we determined that the renal infiltrates of T cells and, unexpectedly, TLR and NLR receptors, are not required to generate Leptospira-induced renal fibrosis. We also show that the iNOS enzyme, known to play a role in Leptospira-induced interstitial nephritis, also plays a role in the induction of renal fibrosis.
Conclusion/significance: To our knowledge, this work provides the first experimental murine model of sustained renal fibrosis induced by a chronic bacterial infection that may be peculiar, since it does not rely on TLR or NLR receptors. This model may prove useful to test future therapeutic strategies to combat Leptospira-induced renal lesions.
Conflict of interest statement
The authors declared that they have no competing interests.
Figures





References
-
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. (2003) Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3: 757–771. - PubMed
-
- Mc IW, Montgomery GL (1952) Renal lesions in Leptospira canicola infection in dogs. J Pathol Bacteriol 64: 145–160. - PubMed
-
- Atasoyu EM, Turhan V, Unver S, Evrenkaya TR, Yildirim S (2005) A case of leptospirosis presenting with end-stage renal failure. Nephrol Dial Transplant 20: 2290–2292. - PubMed
-
- Yang CW (2007) Leptospirosis in Taiwan–an underestimated infectious disease. Chang Gung Med J 30: 109–115. - PubMed
-
- Bandeira M, Santos CS, de Azevedo EC, Soares LM, Macedo JO, et al. (2011) Attenuated nephritis in inducible nitric oxide synthase knockout C57BL/6 mice and pulmonary hemorrhage in CB17 SCID and recombination activating gene 1 knockout C57BL/6 mice infected with Leptospira interrogans. Infect Immun 79: 2936–2940. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

