Leptospira interrogans binds to cadherins
- PMID: 24498454
- PMCID: PMC3907533
- DOI: 10.1371/journal.pntd.0002672
Leptospira interrogans binds to cadherins
Abstract
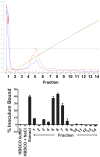
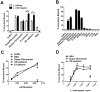
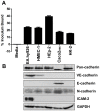
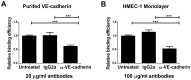
Leptospirosis, caused by pathogenic species of Leptospira, is the most widespread zoonosis and has emerged as a major public health problem worldwide. The adhesion of pathogenic Leptospira to host cells, and to extracellular matrix (ECM) components, is likely to be necessary for the ability of leptospires to penetrate, disseminate and persist in mammalian host tissues. Previous work demonstrated that pathogenic L. interrogans binds to host cells more efficiently than to ECM. Using two independent screening methods, mass spectrometry and protein arrays, members of the cadherin family were identified as potential L. interrogans receptors on mammalian host surfaces. We focused our investigation on vascular endothelial (VE)-cadherin, which is widely expressed on endothelia and is primarily responsible for endothelial cell-cell adhesion. Monolayers of EA.hy926 and HMEC-1 endothelial cells produce VE-cadherin, bind L. interrogans in vitro, and are disrupted upon incubation with the bacteria, which may reflect the endothelial damage seen in vivo. Dose-dependent and saturable binding of L. interrogans to the purified VE-cadherin receptor was demonstrated and pretreatment of purified receptor or endothelial cells with function-blocking antibody against VE-cadherin significantly inhibited bacterial attachment. The contribution of VE-cadherin to leptospiral adherence to host endothelial cell surfaces is biologically significant because VE-cadherin plays an important role in maintaining the barrier properties of the vasculature. Attachment of L. interrogans to the vasculature via VE-cadherin may result in vascular damage, facilitating the escape of the pathogen from the bloodstream into different tissues during disseminated infection, and may contribute to the hemorrhagic manifestations of leptospirosis. This work is first to describe a mammalian cell surface protein as a receptor for L. interrogans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Adler B, de la Pena Moctezuma A (2010) Leptospira and leptospirosis. Vetrinary Microbiology 140: 287–296. - PubMed
-
- Ballard SA, Williamson M, Adler B, Vinh T, Faine S (1986) Interactions of virulent and avirulent leptospires with primary cultures of renal epithelial cells. J Med Microbiol 21: 59–67. - PubMed
-
- Ito T, Yanagawa R (1987) Leptospiral attachment to extracellular matrix of mouse fibroblast (L929) cells. Vet Microbiol 15: 89–96. - PubMed
-
- Merien F, Truccolo J, Rougier Y, Baranton G, Perolat P (1998) In vivo apoptosis of hepatocytes in guinea pigs infected with Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol Lett 169: 95–102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

