STAT2 and IRF9: Beyond ISGF3
- PMID: 24498542
- PMCID: PMC3906322
- DOI: 10.4161/jkst.27521
STAT2 and IRF9: Beyond ISGF3
Abstract
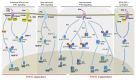
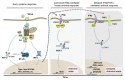
Cytokine signaling is mediated by the combinatorial usage of seven STAT proteins that form homo- or heterodimers involved in the regulation of specific transcriptional programs. Among STATs, STAT2 is classically known to dimerize with STAT1 and together with IRF9 forms the ISGF3 transcription factor complex that has long been considered a hallmark of activation by type I and type III interferons. However, accumulating evidence reveal distinct facets of STAT2 and IRF9 activity mediated by the segregation in alternative STAT1-independent complexes/pathways that are thought to trigger different transcriptional programs. The goal of this review is to summarize our current knowledge of the stimuli, regulatory mechanisms, and function of these alternative pathways.
Keywords: IRF9; ISGF3; ISGs; STAT; TNFα; gene expression; interferon; signaling; synergism.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
