Biochemical phenotype of a common disease-causing mutation and a possible therapeutic approach for the phosphomannomutase 2-associated disorder of glycosylation
- PMID: 24498599
- PMCID: PMC3893156
- DOI: 10.1002/mgg3.3
Biochemical phenotype of a common disease-causing mutation and a possible therapeutic approach for the phosphomannomutase 2-associated disorder of glycosylation
Abstract
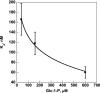
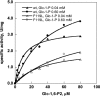
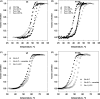
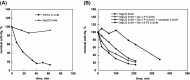
Phosphomannomutase 2 (PMM2) deficiency represents the most frequent type of congenital disorders of glycosylation. For this disease there is no cure at present. The complete loss of phosphomannomutase activity is probably not compatible with life and people affected carry at least one allele with residual activity. We characterized wild-type PMM2 and its most common hypomorphic mutant, p.F119L, which is associated with a severe phenotype of the disease. We demonstrated that active species is the dimeric enzyme and that the mutation weakens the quaternary structure and, at the same time, affects the activity and the stability of the enzyme. We demonstrated that ligand binding stabilizes both proteins, wild-type and F119L-PMM2, and promotes subunit association in vitro. The strongest effects are observed with glucose-1,6-bisphosphate (Glc-1,6-P2) or with monophosphate glucose in the presence of vanadate. This finding offers a new approach for the treatment of PMM2 deficiency. We propose to enhance Glc-1,6-P2 concentration either acting on the metabolic pathways that control its synthesis and degradation or exploiting prodrugs that are able to cross membranes.
Keywords: Congenital disorders of glycosylation; N-glycosylation; glucose 1,6-bisphosphate; phosphomannomutase.
Figures
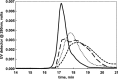
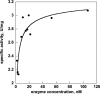
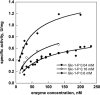
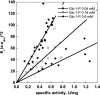

References
-
- Benito JM, Garcia Fernandez JM, Ortiz Mellet C. Pharmacological chaperone therapy for Gaucher disease: a patent review. Exp. Opin. Ther. Pat. 2011;21:885–903. - PubMed
-
- Borkowski T, Orlewska C, Slominska EM, Yuen A, Lipinski M, Rybakowska I, et al. Pharmacological inhibition of AMP-deaminase in rat cardiac myocytes. Nucleosides Nucleotides Nucleic Acids. 2008;27:867–871. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

