Computational replication of the abnormal secondary kinetic isotope effects in a hydride transfer reaction in solution with a motion assisted H-tunneling model
- PMID: 24498946
- PMCID: PMC3985929
- DOI: 10.1021/jo402650a
Computational replication of the abnormal secondary kinetic isotope effects in a hydride transfer reaction in solution with a motion assisted H-tunneling model
Abstract
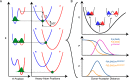
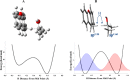
We recently reported abnormal secondary deuterium kinetic isotope effects (2° KIEs) for hydride transfer reactions from alcohols to carbocations in acetonitrile (Chem. Comm. 2012, 48, 11337). Experimental 2° KIE values were found to be inflated on the 9-C position in the xanthylium cation but deflated on the β-C position in 2-propanol with respect to the values predicted by the semi-classical transition-state theory. No primary (1°) isotope effect on 2° KIEs was observed. Herein, the KIEs were replicated by the Marcus-like H-tunneling model that requires a longer donor-acceptor distance (DAD) in a lighter isotope transfer process. The 2° KIEs for a range of potential tunneling-ready-states (TRSs) of different DADs were calculated and fitted to the experiments to find the TRS structure. The observed no effect of 1° isotope on 2° KIEs is explained in terms of the less sterically hindered TRS structure so that the change in DAD due to the change in 1° isotope does not significantly affect the reorganization of the 2° isotope and hence the 2° KIE. The effect of 1° isotope on 2° KIEs may be expected to be more pronounced and thus observable in reactions occurring in restrictive environments such as the crowded and relatively rigid active site of enzymes.
Figures

Similar articles
-
Computational Replication of the Primary Isotope Dependence of Secondary Kinetic Isotope Effects in Solution Hydride-Transfer Reactions: Supporting the Isotopically Different Tunneling Ready State Conformations.J Phys Chem A. 2016 Jun 30;120(25):4277-84. doi: 10.1021/acs.jpca.6b03571. Epub 2016 Jun 20. J Phys Chem A. 2016. PMID: 27232375
-
Steric effects on the primary isotope dependence of secondary kinetic isotope effects in hydride transfer reactions in solution: caused by the isotopically different tunneling ready state conformations?J Am Chem Soc. 2015 May 27;137(20):6653-61. doi: 10.1021/jacs.5b03085. Epub 2015 May 15. J Am Chem Soc. 2015. PMID: 25941865
-
Replication of the Enzymatic Temperature Dependency of the Primary Hydride Kinetic Isotope Effects in Solution: Caused by the Protein-Controlled Rigidity of the Donor-Acceptor Centers?Biochemistry. 2019 Oct 1;58(39):4035-4046. doi: 10.1021/acs.biochem.9b00574. Epub 2019 Sep 13. Biochemistry. 2019. PMID: 31478638
-
Understanding Biological Hydrogen Transfer Through the Lens of Temperature Dependent Kinetic Isotope Effects.Acc Chem Res. 2018 Sep 18;51(9):1966-1974. doi: 10.1021/acs.accounts.8b00226. Epub 2018 Aug 28. Acc Chem Res. 2018. PMID: 30152685 Free PMC article. Review.
-
Large Isotope Effects in Organometallic Chemistry.Chemistry. 2021 Oct 25;27(60):14800-14815. doi: 10.1002/chem.202102189. Epub 2021 Sep 23. Chemistry. 2021. PMID: 34347912 Review.
Cited by
-
Structural Effects on the Temperature Dependence of Hydride Kinetic Isotope Effects of the NADH/NAD+ Model Reactions in Acetonitrile: Charge-Transfer Complex Tightness Is a Key.J Org Chem. 2024 Mar 1;89(5):3184-3193. doi: 10.1021/acs.joc.3c02562. Epub 2024 Feb 16. J Org Chem. 2024. PMID: 38364859 Free PMC article.
-
Study of the Effects of Remote Heavy Group Vibrations on the Temperature Dependence of Hydride Kinetic Isotope Effects of the NADH/NAD+ Model Reactions.ACS Omega. 2024 Apr 24;9(18):20593-20600. doi: 10.1021/acsomega.4c02383. eCollection 2024 May 7. ACS Omega. 2024. PMID: 38737086 Free PMC article.
-
Correlation of temperature dependence of hydride kinetic isotope effects with donor-acceptor distances in two solvents of different polarities.Org Biomol Chem. 2023 Jun 21;21(24):5090-5097. doi: 10.1039/d3ob00718a. Org Biomol Chem. 2023. PMID: 37278324 Free PMC article.
References
-
- More O’Ferrall R. A. J. Phy. Org. Chem. 2010, 23, 559.
-
- Bell R. P.The Tunnel Effect in Chemistry; Chapman & Hall: London, New York, 1980.
-
- Kohen A.; Cannio R.; Bartolucci S.; Klinman J. P. Nature 1999, 399, 496. - PubMed
-
- Basran J.; Sutcliffe M. J.; Scrutton N. S. Biochemistry 1999, 38, 3218. - PubMed
-
- Harris R. J.; Meskys R.; Sutcliffe M. J.; Scrutton N. S. Biochemistry 2000, 39, 1189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

