Toll like receptor (TLR)-4 as a regulator of peripheral endogenous opioid-mediated analgesia in inflammation
- PMID: 24499354
- PMCID: PMC3922964
- DOI: 10.1186/1744-8069-10-10
Toll like receptor (TLR)-4 as a regulator of peripheral endogenous opioid-mediated analgesia in inflammation
Abstract
Background: Leukocytes containing opioid peptides locally control inflammatory pain. In the early phase of complete Freund's adjuvant (CFA)-induced hind paw inflammation, formyl peptides (derived e.g. from Mycobacterium butyricum) trigger the release of opioid peptides from neutrophils contributing to tonic basal antinociception. In the later phase we hypothesized that toll-like-receptor-(TLR)-4 activation of monocytes/macrophages triggers opioid peptide release and thereby stimulates peripheral opioid-dependent antinociception.
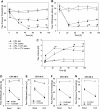
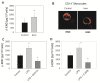
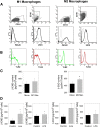
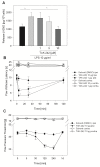
Results: In Wistar rats with CFA hind paw inflammation in the later inflammatory phase (48-96 h) systemic leukocyte depletion by cyclophosphamide (CTX) or locally injected naloxone (NLX) further decreased mechanical and thermal nociceptive thresholds. In vitro β-endorphin (β-END) content increased during human monocyte differentiation as well as in anti-inflammatory CD14+CD16- or non-classical M2 macrophages. Monocytes expressing TLR4 dose-dependently released β-END after stimulation with lipopolysaccharide (LPS) dependent on intracellular calcium. Despite TLR4 expression proinflammatory M1 and anti-inflammatory M2 macrophages only secreted opioid peptides in response to ionomycin, a calcium ionophore. Intraplantar injection of LPS as a TLR4 agonist into the inflamed paw elicited an immediate opioid- and dose-dependent antinociception, which was blocked by TAK-242, a small-molecule inhibitor of TLR4, or by peripheral applied NLX. In the later phase LPS lowered mechanical and thermal nociceptive thresholds. Furthermore, local peripheral TLR4 blockade worsened thermal and mechanical nociceptive pain thresholds in CFA inflammation.
Conclusion: Endogenous opioids from monocytes/macrophages mediate endogenous antinociception in the late phase of inflammation. Peripheral TLR4 stimulation acts as a transient counter-regulatory mechanism for inflammatory pain in vivo, and increases the release of opioid peptides from monocytes in vitro. TLR4 antagonists as new treatments for sepsis and neuropathic pain might unexpectedly transiently enhance pain by impairing peripheral opioid analgesia.
Figures



Similar articles
-
Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils.PLoS Pathog. 2009 Apr;5(4):e1000362. doi: 10.1371/journal.ppat.1000362. Epub 2009 Apr 3. PLoS Pathog. 2009. PMID: 19343210 Free PMC article.
-
Long-term antinociception by electroacupuncture is mediated via peripheral opioid receptors in free-moving rats with inflammatory hyperalgesia.Eur J Pain. 2013 Nov;17(10):1447-57. doi: 10.1002/j.1532-2149.2013.00325.x. Epub 2013 May 6. Eur J Pain. 2013. PMID: 23649949
-
Tissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociception.Anesthesiology. 2004 Jul;101(1):204-11. doi: 10.1097/00000542-200407000-00031. Anesthesiology. 2004. PMID: 15220792
-
Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility.Front Immunol. 2020 Jul 8;11:1455. doi: 10.3389/fimmu.2020.01455. eCollection 2020. Front Immunol. 2020. PMID: 32733481 Free PMC article. Review.
-
Therapeutic Developments Targeting Toll-like Receptor-4-Mediated Neuroinflammation.ChemMedChem. 2016 Jan 19;11(2):154-65. doi: 10.1002/cmdc.201500188. Epub 2015 Jul 1. ChemMedChem. 2016. PMID: 26136385 Free PMC article. Review.
Cited by
-
Corneal pain and experimental model development.Prog Retin Eye Res. 2019 Jul;71:88-113. doi: 10.1016/j.preteyeres.2018.11.005. Epub 2018 Nov 16. Prog Retin Eye Res. 2019. PMID: 30453079 Free PMC article. Review.
-
Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug.Arthritis Res Ther. 2020 Jan 23;22(1):16. doi: 10.1186/s13075-020-2097-2. Arthritis Res Ther. 2020. PMID: 31973752 Free PMC article.
-
Effects of Pulsed Radiofrequency Current and Thermal Condition on the Expression of β-Endorphin in Human Monocytic Cells.NeuroSci. 2025 Jul 21;6(3):67. doi: 10.3390/neurosci6030067. NeuroSci. 2025. PMID: 40700132 Free PMC article.
-
Decrease of IL-1β and TNF in the Spinal Cord Mediates Analgesia Produced by Ankle Joint Mobilization in Complete Freund Adjuvant-Induced Inflammation Mice Model.Front Physiol. 2022 Jan 14;12:816624. doi: 10.3389/fphys.2021.816624. eCollection 2021. Front Physiol. 2022. PMID: 35095573 Free PMC article.
-
The anti-inflammatory and analgesic properties of prosopis chilenses in rats.Int J Health Sci (Qassim). 2015 Jul;9(3):265-71. Int J Health Sci (Qassim). 2015. PMID: 26609291 Free PMC article.
References
-
- Rittner HL, Machelska H, Stein C. Leukocytes in the regulation of pain and analgesia. J Leukoc Biol. 2005;79:1215–1222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

