A new haemocyanin in cuttlefish (Sepia officinalis) eggs: sequence analysis and relevance during ontogeny
- PMID: 24499521
- PMCID: PMC3945787
- DOI: 10.1186/2041-9139-5-6
A new haemocyanin in cuttlefish (Sepia officinalis) eggs: sequence analysis and relevance during ontogeny
Abstract
Background: Haemocyanin is the respiratory protein of most of the Mollusca. In cephalopods and gastropods at least two distinct isoforms are differentially expressed. However, their physiological purpose is unknown. For the common cuttlefish Sepia officinalis, three isoforms are known so far, whereas for only two of them the complete mRNA sequences are available. In this study, we sequenced the complete mRNA of the third haemocyanin isoform and measured the relative expression of all three isoforms during embryogenesis to reveal a potential ontogenetic relevance.
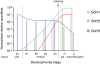
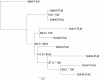
Results: The cDNA of isoform 3 clearly correlates to the known Sepia officinalis haemocyanin subunits consisting of eight functional units and an internal duplicated functional unit d. Our molecular phylogenetic analyses reveal the third isoform representing a potentially ancestral haemocyanin isoform, and the analyses of the expression of haemocyanin type 3 reveal that haemocyanin type 3 only can be observed within eggs and during early development. Isoforms 1 and 2 are absent at these stages. After hatching, isoform 3 is downregulated, and isoform 1 and 2 are upregulated.
Conclusions: Our study clearly shows an embryonic relevance of the third isoform, which will be further discussed in the light of the changes in the physiological function of haemocyanin during ontogeny. Taken together with the fact that it could also be the isoform closest related to the common ancestor of cuttlefish haemocyanin, the phylogeny of cuttlefish haemocyanin may be recapitulated during its ontogeny.
Figures



Similar articles
-
Influence of temperature, hypercapnia, and development on the relative expression of different hemocyanin isoforms in the common cuttlefish Sepia officinalis.J Exp Zool A Ecol Genet Physiol. 2012 Dec;317(8):511-23. doi: 10.1002/jez.1743. Epub 2012 Jul 12. J Exp Zool A Ecol Genet Physiol. 2012. PMID: 22791630
-
Haemocyanin mRNA from arthropod and mollusc origin. Evidence for a multi-unit structure in mollusc haemocyanin mRNA.Biochem J. 1986 Jan 1;233(1):253-7. doi: 10.1042/bj2330253. Biochem J. 1986. PMID: 3954728 Free PMC article.
-
A difference in timing for the onset of visual and chemosensory systems during embryonic development in two closely related cuttlefish species.Dev Psychobiol. 2019 Nov;61(7):1014-1021. doi: 10.1002/dev.21868. Epub 2019 Jun 6. Dev Psychobiol. 2019. PMID: 31172508
-
Diversity of Light Sensing Molecules and Their Expression During the Embryogenesis of the Cuttlefish (Sepia officinalis).Front Physiol. 2020 Sep 29;11:521989. doi: 10.3389/fphys.2020.521989. eCollection 2020. Front Physiol. 2020. PMID: 33117186 Free PMC article.
-
Digestive enzyme ratios are good indicators of hatchling yolk reserve and digestive gland maturation in early life stages of cuttlefish Sepia officinalis L.: application of these new tools in ecology and aquaculture.J Comp Physiol B. 2018 Jan;188(1):57-76. doi: 10.1007/s00360-017-1115-4. Epub 2017 Jul 10. J Comp Physiol B. 2018. PMID: 28691154
Cited by
-
Molluscan hemocyanin: structure, evolution, and physiology.Biophys Rev. 2018 Apr;10(2):191-202. doi: 10.1007/s12551-017-0349-4. Epub 2017 Dec 12. Biophys Rev. 2018. PMID: 29235083 Free PMC article. Review.
-
Positive selection in octopus haemocyanin indicates functional links to temperature adaptation.BMC Evol Biol. 2015 Jul 5;15:133. doi: 10.1186/s12862-015-0411-4. BMC Evol Biol. 2015. PMID: 26142723 Free PMC article.
-
The Evolution of Hemocyanin Genes in Caenogastropoda: Gene Duplications and Intron Accumulation in Highly Diverse Gastropods.J Mol Evol. 2021 Dec;89(9-10):639-655. doi: 10.1007/s00239-021-10036-y. Epub 2021 Nov 10. J Mol Evol. 2021. PMID: 34757470 Free PMC article.
-
Cryo-EM reveals the asymmetric assembly of squid hemocyanin.IUCrJ. 2019 Apr 5;6(Pt 3):426-437. doi: 10.1107/S205225251900321X. eCollection 2019 May 1. IUCrJ. 2019. PMID: 31098023 Free PMC article.
-
The evolution of hemocyanin genes in Tectipleura: a multitude of conserved introns in highly diverse gastropods.BMC Ecol Evol. 2021 Mar 4;21(1):36. doi: 10.1186/s12862-021-01763-3. BMC Ecol Evol. 2021. PMID: 33663373 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

