Efficacy of black cohosh (Cimicifuga racemosa L.) in treating early symptoms of menopause: a randomized clinical trial
- PMID: 24499633
- PMCID: PMC4029542
- DOI: 10.1186/1749-8546-8-20
Efficacy of black cohosh (Cimicifuga racemosa L.) in treating early symptoms of menopause: a randomized clinical trial
Abstract
Background: This study aims to evaluate the efficacy of Black cohosh (Cimicifuga racemosa L.) in treating early menopausal symptoms.
Methods: This randomized, double-blind, placebo-controlled clinical trial was conducted on 84 early post-menopausal participants with Greene climacteric scale (GCS) scores of 15 to 42, who were referred to two public health care centers in Tehran, Iran, in 2011-2012. The participants were randomly allocated into treatment (6.5 mg of dried extract of Black cohosh roots daily) and control (placebo) groups with a ratio of 1:1. The participants took one tablet per day for 8 weeks. The GCS scores were recorded at baseline, and after 4 and 8 weeks of treatment. Data analysis was carried out using a general linear model with repeated measures with SPSS software. The level of significance was set at P < 0.05.
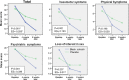
Results: There was no loss to follow-up during the 8 weeks of treatment. The GCS total score (primary outcome) in the treatment group was significantly lower than that in the control group at both week 4 [adjusted mean difference: -7.8 (95% confidence interval: -11.1 to -4.4)] and week 8 [-12.9 (-16.2 to -9.3)]. The treatment group showed significantly more improvement than the control group in all GCS subscale scores (vasomotor, psychiatric, physical, and sexual symptoms; secondary outcomes). The differences between the treatment and control groups at week 8 were significantly higher (P < 0.001) than those at week 4 in terms of the total scores and the vasomotor and psychiatric subscale scores. No side effects were reported.
Conclusions: Black cohosh reduced the GCS total score and all GCS subscale scores (vasomotor, psychiatric, physical, and sexual symptoms) during 4 and 8 weeks of treatment.
Clinical trial registration: This study was approved (Code 9061) by the Ethics Committee of Tabriz University of Medical Sciences and registered at the Iranian Registry of Clinical Trials with IRCT201107186709N4 on 15 January 2012.
Figures
Similar articles
-
Ethnobotany, Phytochemistry, Traditional and Modern Uses of Actaea racemosa L. (Black cohosh): A Review.Adv Exp Med Biol. 2021;1308:403-449. doi: 10.1007/978-3-030-64872-5_24. Adv Exp Med Biol. 2021. PMID: 33861455 Review.
-
Randomized, double-blind, placebo-controlled trial of Cimicifuga racemosa (black cohosh) in women with anxiety disorder due to menopause.J Clin Psychopharmacol. 2009 Oct;29(5):478-83. doi: 10.1097/JCP.0b013e3181b2abf2. J Clin Psychopharmacol. 2009. PMID: 19745648 Free PMC article. Clinical Trial.
-
Effect of black cohosh (cimicifuga racemosa) on vasomotor symptoms in postmenopausal women: a randomized clinical trial.J Caring Sci. 2013 Jun 1;2(2):105-13. doi: 10.5681/jcs.2013.013. eCollection 2013 Jun. J Caring Sci. 2013. PMID: 25276716 Free PMC article.
-
Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: a randomized trial.Ann Intern Med. 2006 Dec 19;145(12):869-79. doi: 10.7326/0003-4819-145-12-200612190-00003. Ann Intern Med. 2006. PMID: 17179056 Clinical Trial.
-
[Efficacy and safety of Black cohosh (Actaea/Cimicifuga racemosa) in the treatment of vasomotor symptoms--review of clinical trials].Ginekol Pol. 2008 Apr;79(4):287-96. Ginekol Pol. 2008. PMID: 18592868 Review. Polish.
Cited by
-
Ethnobotany, Phytochemistry, Traditional and Modern Uses of Actaea racemosa L. (Black cohosh): A Review.Adv Exp Med Biol. 2021;1308:403-449. doi: 10.1007/978-3-030-64872-5_24. Adv Exp Med Biol. 2021. PMID: 33861455 Review.
-
Effects of Bushen Tianjing Recipe in a rat model of tripterygium glycoside-induced premature ovarian failure.Chin Med. 2017 Apr 24;12:10. doi: 10.1186/s13020-017-0131-3. eCollection 2017. Chin Med. 2017. PMID: 28439292 Free PMC article.
-
Benefits of Black Cohosh (Cimicifuga racemosa) for Women Health: An Up-Close and In-Depth Review.Pharmaceuticals (Basel). 2022 Feb 23;15(3):278. doi: 10.3390/ph15030278. Pharmaceuticals (Basel). 2022. PMID: 35337076 Free PMC article. Review.
-
The Effect of Lactobacillus acidophilus YT1 (MENOLACTO) on Improving Menopausal Symptoms: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial.J Clin Med. 2020 Jul 9;9(7):2173. doi: 10.3390/jcm9072173. J Clin Med. 2020. PMID: 32660010 Free PMC article.
-
Modulation of the Proliferative Pathway, Neuroinflammation and Pain in Endometriosis.Int J Mol Sci. 2023 Jul 21;24(14):11741. doi: 10.3390/ijms241411741. Int J Mol Sci. 2023. PMID: 37511500 Free PMC article.
References
-
- The North American Menopause Society. Menopause Practice: A Clinician’s Guide. 4. Ohio: NA MENOP; 2010.
-
- Shifren JL. In: Berek and Novak’s Gynecology. 14. Berek JS, editor. Philadelphia: Lippincott Williams and Wilkins; 2007. Menopause; pp. 1323–1339.
-
- Low Dog T, Riley D, Carter T. An integrative approach to menopause. Altern ther Health Med. 2001;8:45–55. - PubMed
-
- Impey L, Child T. Obstetrics and Gynecology. Oxford: Blackwell Science; 1999.
LinkOut - more resources
Full Text Sources
Other Literature Sources