Alveolar progenitor and stem cells in lung development, renewal and cancer
- PMID: 24499815
- PMCID: PMC4013278
- DOI: 10.1038/nature12930
Alveolar progenitor and stem cells in lung development, renewal and cancer
Abstract
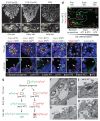
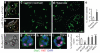
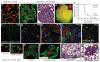
Alveoli are gas-exchange sacs lined by squamous alveolar type (AT) 1 cells and cuboidal, surfactant-secreting AT2 cells. Classical studies suggested that AT1 arise from AT2 cells, but recent studies propose other sources. Here we use molecular markers, lineage tracing and clonal analysis to map alveolar progenitors throughout the mouse lifespan. We show that, during development, AT1 and AT2 cells arise directly from a bipotent progenitor, whereas after birth new AT1 cells derive from rare, self-renewing, long-lived, mature AT2 cells that produce slowly expanding clonal foci of alveolar renewal. This stem-cell function is broadly activated by AT1 injury, and AT2 self-renewal is selectively induced by EGFR (epidermal growth factor receptor) ligands in vitro and oncogenic Kras(G12D) in vivo, efficiently generating multifocal, clonal adenomas. Thus, there is a switch after birth, when AT2 cells function as stem cells that contribute to alveolar renewal, repair and cancer. We propose that local signals regulate AT2 stem-cell activity: a signal transduced by EGFR-KRAS controls self-renewal and is hijacked during oncogenesis, whereas another signal controls reprogramming to AT1 fate.
Figures


References
-
- Bertalanffy FD, Leblond CP. Structure of respiratory tissue. Lancet. 1955;269:1365–1368. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol. 2011;27:493–512. - PubMed
-
- Adamson IY, Bowden DH. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest. 1975;32:736–745. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

