A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder
- PMID: 24500245
- PMCID: PMC5360105
- DOI: 10.4088/JCP.13m08591
A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder
Abstract
Objective: To examine the comparative antidepressant efficacy of S-adenosyl-L-methionine (SAMe) and escitalopram in a placebo-controlled, randomized, double-blind clinical trial.
Method: One hundred eighty-nine outpatients (49.7% female, mean [SD] age = 45 [15] years) with DSM-IV-diagnosed major depressive disorder (MDD) were recruited from April 13, 2005, to December 22, 2009, at the Massachusetts General Hospital and at Butler Hospital. Patients were randomized for 12 weeks to SAMe 1,600-3,200 mg/d, escitalopram 10-20 mg/d, or placebo. Doses were escalated at 6 weeks in the event of nonresponse. The main outcome measure was the 17-item Hamilton Depression Rating Scale (HDRS-17). Tolerability was assessed by the Systematic Assessment for Treatment of Emergent Events-Specific Inquiry (SAFTEE-SI).
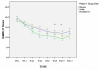
Results: All 3 treatment arms demonstrated a significant improvement of about 5-6 points in HDRS-17 scores (P < .001 for all), and no significant differences were observed between the treatment arms (P > .05 for all). Response rates in the intent-to-treat sample were 36% for SAMe, 34% for escitalopram, and 30% for placebo. Remission rates were 28% for SAMe, 28% for escitalopram, and 17% for placebo. No comparisons between treatment groups attained significance (P > .05 for all). Tolerability was good, with gastrointestinal side effects (19% for stomach discomfort and 20% for diarrhea) as the most common in the SAMe arm. Significant differences were observed between treatment groups for dizziness, anorgasmia, diminished mental acuity, and hot flashes (P < .05 for all).
Conclusions: The results fail to support an advantage over placebo for either the investigational treatment SAMe or the standard treatment escitalopram for MDD.
Trial registration: ClinicalTrials.gov identifier: NCT00101452.
© Copyright 2013 Physicians Postgraduate Press, Inc.
Figures
Comment in
-
Failed studies should not be used to malign good treatments.J Clin Psychiatry. 2014 Nov;75(11):e1328. doi: 10.4088/JCP.14lr09266. J Clin Psychiatry. 2014. PMID: 25470102 No abstract available.
-
Dr. Mischoulon and colleagues reply.J Clin Psychiatry. 2014 Nov;75(11):e1328-9. doi: 10.4088/JCP.14lr09266a. J Clin Psychiatry. 2014. PMID: 25470103 No abstract available.
References
-
- Spillmann M, Fava M. S-adenosyl-methionine (ademethionine) in psychiatric disorders. CNS Drugs. 1996;6(6):416–425.
-
- Baldessarini RJ. The neuropharmacology of S-adenosyl-L-methionine. Am J Medicine. 1987;83(5A):95–103. - PubMed
-
- Papakostas GI, Alpert JE, Fava M. S-adenosyl-methionine in depression: a comprehensive review of the literature. Curr Psychiatry Rep. 2003;5(6):460–466. - PubMed
-
- Papakostas GI. Evidence for S-adenosyl-L-methionine (SAM-e) for the treatment of major depressive disorder. J Clin Psychiatry. 2009;70(Suppl 5):18–22. - PubMed
-
- Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl Methionine (SAMe) Augmentation of Serotonin Reuptake Inhibitors (SRIs) for SRI- Non-responders with Major Depressive Disorder: A Double-blind, Randomized Clinical Trial. American J Psychiatry. 2010;167(8):942–948. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous