Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo
- PMID: 24500276
- PMCID: PMC4075037
- DOI: 10.1038/ncomms4262
Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo
Abstract
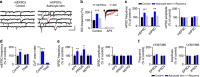
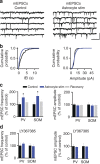
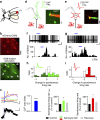
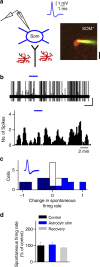
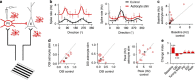
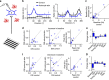
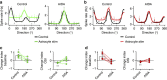
Astrocytes play important roles in synaptic transmission and plasticity. Despite in vitro evidence, their causal contribution to cortical network activity and sensory information processing in vivo remains unresolved. Here we report that selective photostimulation of astrocytes with channelrhodopsin-2 in primary visual cortex enhances both excitatory and inhibitory synaptic transmission, through the activation of type 1a metabotropic glutamate receptors. Photostimulation of astrocytes in vivo increases the spontaneous firing of parvalbumin-positive (PV(+)) inhibitory neurons, while excitatory and somatostatin-positive (SOM(+)) neurons show either an increase or decrease in their activity. Moreover, PV(+) neurons show increased baseline visual responses and reduced orientation selectivity to visual stimuli, whereas excitatory and SOM(+) neurons show either increased or decreased baseline visual responses together with complementary changes in orientation selectivity. Therefore, astrocyte activation, through the dual control of excitatory and inhibitory drive, influences neuronal integrative features critical for sensory information processing.
Figures


References
-
- Perea G., Navarrete M. & Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32, 421–431 (2009). - PubMed
-
- Di Castro M. A. et al.. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284 (2011). - PubMed
-
- Panatier A. et al.. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

