Transcriptional control of a whole chromosome: emerging models for dosage compensation
- PMID: 24500429
- PMCID: PMC4342042
- DOI: 10.1038/nsmb.2763
Transcriptional control of a whole chromosome: emerging models for dosage compensation
Abstract
Males and females of many animal species differ in their sex-chromosome karyotype, and this creates imbalances between X-chromosome and autosomal gene products that require compensation. Although distinct molecular mechanisms have evolved in three highly studied systems, they all achieve coordinate regulation of an entire chromosome by differential RNA-polymerase occupancy at X-linked genes. High-throughput genome-wide methods have been pivotal in driving the latest progress in the field. Here we review the emerging models for dosage compensation in mammals, flies and nematodes, with a focus on mechanisms affecting RNA polymerase II activity on the X chromosome.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
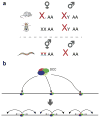
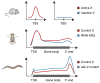

References
-
- Charlesworth D, Charlesworth B, Marais G. Steps in the evolution of sex chromosomes. Heredity (Edinb) 2005;95:118–128. - PubMed
-
- Lucchesi JC, Kelly WG, Panning B. Chromatin remodeling in dosage compensation. Annu Rev Genet. 2005;39:615–651. - PubMed
-
- Kruesi WS, Core LJ, Waters CT, Lis JT, Meyer BJ. Condensin controls recruitment of RNA polymerase II to achieve nematode X-chromosome dosage compensation. Elife. 2013;2:e00808. Reports genome-wide analyses demonstrating repression of RNA-polymerase recruitment to X-linked genes as the molecular mechanism for C. elegans dosage compensation. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

