Tiagabine add-on for drug-resistant partial epilepsy
- PMID: 24500879
- PMCID: PMC6718214
- DOI: 10.1002/14651858.CD001908.pub3
Tiagabine add-on for drug-resistant partial epilepsy
Abstract
Background: Epilepsy is a common neurological condition that affects almost 0.5% to 1% of the population. Nearly 30% of people with epilepsy are resistant to currently available drugs. Tiagabine is one of the newer antiepileptic drugs; its effects as an adjunct (add-on) to standard drugs are assessed in this review.
Objectives: To evaluate the effects of add-on treatment with tiagabine on seizures, adverse effects, cognition and quality of life for people with drug-resistant localisation-related seizures.
Search methods: This is an updated version of the original Cochrane review published in 2012 (Issue 5). We searched the Cochrane Epilepsy Group Specialised Register (November 2013), the Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 10) and MEDLINE (1946 to November 2013). No language restrictions were imposed. We also contacted the manufacturers of tiagabine and experts in the field to seek any ongoing or unpublished studies.
Selection criteria: Randomised placebo-controlled add-on trials of people of any age with localisation-related seizures in which an adequate method of concealment of randomisation was used were included. The studies could be double-blind, single-blind or unblinded and of parallel or cross-over design. They had to have a minimum treatment period of eight weeks. Trials using an active drug control group were also included.
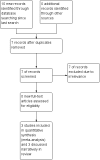
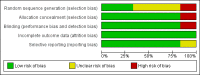
Data collection and analysis: Two review authors independently selected trials for inclusion and extracted data. Disagreements were resolved by discussion. Outcomes investigated included 50% or greater reduction in seizure frequency, treatment withdrawal, adverse effects, effects on cognition and quality of life. The primary analyses were performed by intention-to-treat. Worst-case and best-case analyses were calculated for seizure outcomes. Dose response was evaluated in regression models. Risk of bias in each study was assessed by two review authors using the Cochrane 'Risk of bias' tool.
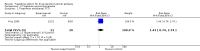
Main results: Four parallel-group and two cross-over group trials were included. The overall risk ratio (RR) with 95% confidence intervals (CIs) for a 50% or greater reduction in seizure frequency (tiagabine vs placebo) was 3.16 (95% CI 1.97 to 5.07). Because of differences in response rates among trials, regression models were unable to provide reliable estimates of response to individual doses. The RR for treatment withdrawal was 1.81 (95% CI 1.25 to 2.62). The 99% CIs for the adverse effects of dizziness, fatigue, nervousness and tremor did not include unity, indicating that they are significantly associated with tiagabine. For cognitive and quality of life outcomes, the limited available data suggested no significant effects on cognition and mood and adjustment. Two of the five studies were judged as having low risk of bias, three studies unclear risk of bias and one study high risk of bias. Overall study quality was rated as high using the GRADE approach.
Authors' conclusions: Tiagabine reduces seizure frequency but is associated with some adverse effects when used as an add-on treatment for people with drug-resistant localisation-related seizures.
Conflict of interest statement
Prof Marson has received payment for speaking and for attending conferences by Sanofi‐Synthelabo, the current manufacturers of tiagabine, and GSK. In addition, a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to University of Liverpool.
Jennifer Pulman and Jane L Hutton have no conflicts of interest.
Figures















Update of
-
Tiagabine add-on for drug-resistant partial epilepsy.Cochrane Database Syst Rev. 2012 May 16;5(5):CD001908. doi: 10.1002/14651858.CD001908.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Feb 05;(2):CD001908. doi: 10.1002/14651858.CD001908.pub3. PMID: 22592677 Free PMC article. Updated.
References
References to studies included in this review
-
- Crawford PM, Meinardi H, Brown S, Rentmeester TW, Pedersen PC, Lassen LC. Tiagabine: efficacy and safety in adjunctive treatment of partial seizures. Epilepsia 2001;42(4):531‐8. - PubMed
-
- Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, Helmstaedter C. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy & Behavior 2005;6(3):373‐81. - PubMed
-
- Kalviainen R, Aikia M, Mervaala E, Saukkonen AM, Pitkanen A, Riekkinen PJ Sr. Long‐term cognitive and EEG effects of tiagabine in drug‐resistant partial epilepsy. Epilepsy Research 1996;25(3):291‐7. - PubMed
- Kalviainen R, Brodie MJ, Duncan J, Chadwick D, Edwards D, Lyby K. A double‐blind, placebo‐controlled trial of tiagabine given three‐times daily as add‐on therapy for refractory partial seizures. Epilepsy Research 1998;30:31‐40. - PubMed
-
- Richens A, Chadwick DW, Duncan JS, Dam M, Gram L, Mikkelsen M, et al. Adjunctive treatment of partial seizures with tiagabine: a placebo‐controlled trial. Epilepsy Research 1995;21(1):37‐42. [MEDLINE: ; CN‐00015195 ‐ CCTR] - PubMed
- Sveinbjornsdottir S, Sander JW, Patsalos PN, Upton D, Thompson PJ, Duncan JS. Neuropsychological effects of tiagabine, a potential new antiepileptic drug. Seizure 1994;3(1):29‐35. - PubMed
-
- Sachdeo RC, Leroy RF, Krauss GL, Drake ME Jr, Green PM, Leppik IE, et al. Tiagabine therapy for complex partial seizures: a dose‐frequency study. Archives of Neurology 1997;54:595‐601. - PubMed
References to studies excluded from this review
-
- Arroyo S, Bootham BR, Brodie MJ, Duncan JS, Duncan R, Nieto M. A randomised open‐label study of tiagabine given two or three times daily in refractory epilepsy. Seizure 2005;14(2):81‐4. - PubMed
-
- Bauer J, Stawowy B, Lenders T, Bettig U, Elger CE. Efficacy and tolerability of tiagabine: results of an add‐on study in patients with refractory partial seizures. Journal of Epilepsy 1995;8(1):83‐6.
-
- Gustavson LE, Boellner SW, Granneman GR, Qian JX, Guenther HJ, El‐Shourbagy T, et al. A single‐dose study to define tiagabine pharmacokinetics in pediatric patients with complex partial seizures. Neurology 1997;48(4):1032‐7. - PubMed
-
- Uldall P, Bulteau C, Pedersen SA, Dulac O, Meinild H, Lassen LC. Single‐blind study of safety, tolerability and preliminary efficacy of tiagabine as adjunctive treatment of children with epilepsy. Epilepsia 1995;36 Suppl(3):S147‐S148.
Additional references
-
- Baker GA, Hesdon M, Marson AG. Quality of life and behavioural outcome measures in randomized controlled trials of antiepileptic drugs: a systematic review of reporting standards. Epilepsia 2000;41(11):1357‐63. - PubMed
-
- Cochrane HC, Baker GA, Chadwick DW. Neuropsychological outcomes in randomized controlled trials of antiepileptic drugs: a systematic review of methodology and reporting standards. Epilepsia 1998;39(10):1088‐97. - PubMed
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the national general practice study of epilepsy. Lancet 1995;346:140‐4. - PubMed
-
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30(4):389‐99. - PubMed
-
- Dodrill CB, Arnett JL, Sommerville KW, Shu V. Cognitive and quality of life effects of differing dosages of tiagabine in epilepsy. Neurology 1997;48(4):1025‐31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

