Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function
- PMID: 24500923
- PMCID: PMC3986846
- DOI: 10.1096/fj.13-237388
Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function
Abstract
Duchenne muscular dystrophy in boys progresses rapidly to severe impairment of muscle function and death in the second or third decade of life. Current supportive therapy with corticosteroids results in a modest increase in strength as a consequence of a general reduction in inflammation, albeit with potential untoward long-term side effects and ultimate failure of the agent to maintain strength. Here, we demonstrate that alternative approaches that rescue defective autophagy in mdx mice, a model of Duchenne muscular dystrophy, with the use of rapamycin-loaded nanoparticles induce a reproducible increase in both skeletal muscle strength and cardiac contractile performance that is not achievable with conventional oral rapamycin, even in pharmacological doses. This increase in physical performance occurs in both young and adult mice, and, surprisingly, even in aged wild-type mice, which sets the stage for consideration of systemic therapies to facilitate improved cell function by autophagic disposal of toxic byproducts of cell death and regeneration.
Keywords: Duchenne; cardiomyopathy; drug delivery; nanomedicine; perfluorocarbon.
Figures




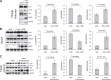

References
-
- Hoffman E. P., Brown R. H., Jr., Kunkel L. M. (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 - PubMed
-
- Moxley R. T., 3rd, Ashwal S., Pandya S., Connolly A., Florence J., Mathews K., Baumbach L., McDonald C., Sussman M., Wade C. (2005) Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 64, 13–20 - PubMed
-
- Markham L. W., Spicer R. L., Khoury P. R., Wong B. L., Mathews K. D., Cripe L. H. (2005) Steroid therapy and cardiac function in Duchenne muscular dystrophy. Pediatr. Cardiol. 26, 768–771 - PubMed
-
- Biggar W. D., Harris V. A., Eliasoph L., Alman B. (2006) Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul. Disord. 16, 249–255 - PubMed
-
- Balaban B., Matthews D. J., Clayton G. H., Carry T. (2005) Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am. J. Phys. Med. Rehab. 84, 843–850 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

