Scavenging iron: a novel mechanism of plant immunity activation by microbial siderophores
- PMID: 24501001
- PMCID: PMC3982770
- DOI: 10.1104/pp.113.233585
Scavenging iron: a novel mechanism of plant immunity activation by microbial siderophores
Abstract
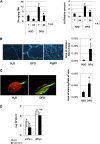
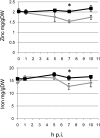
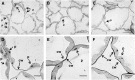
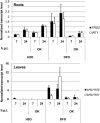
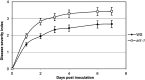
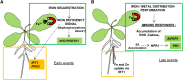
Siderophores are specific ferric iron chelators synthesized by virtually all microorganisms in response to iron deficiency. We have previously shown that they promote infection by the phytopathogenic enterobacteria Dickeya dadantii and Erwinia amylovora. Siderophores also have the ability to activate plant immunity. We have used complete Arabidopsis transcriptome microarrays to investigate the global transcriptional modifications in roots and leaves of Arabidopsis (Arabidopsis thaliana) plants after leaf treatment with the siderophore deferrioxamine (DFO). Physiological relevance of these transcriptional modifications was validated experimentally. Immunity and heavy-metal homeostasis were the major processes affected by DFO. These two physiological responses could be activated by a synthetic iron chelator ethylenediamine-di(o-hydroxyphenylacetic) acid, indicating that siderophores eliciting activities rely on their strong iron-chelating capacity. DFO was able to protect Arabidopsis against the pathogenic bacterium Pseudomonas syringae pv tomato DC3000. Siderophore treatment caused local modifications of iron distribution in leaf cells visible by ferrocyanide and diaminobenzidine-H₂O₂ staining. Metal quantifications showed that DFO causes a transient iron and zinc uptake at the root level, which is presumably mediated by the metal transporter iron regulated transporter1 (IRT1). Defense gene expression and callose deposition in response to DFO were compromised in an irt1 mutant. Consistently, plant susceptibility to D. dadantii was increased in the irt1 mutant. Our work shows that iron scavenging is a unique mechanism of immunity activation in plants. It highlights the strong relationship between heavy-metal homeostasis and immunity.
Figures

References
-
- Andrews SC, Robinson AK, Rodríguez-Quiñones F. (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27: 215–237 - PubMed
-
- Arnaud N, Murgia I, Boucherez J, Briat JF, Cellier F, Gaymard F. (2006) An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. J Biol Chem 281: 23579–23588 - PubMed
-
- Bakker PAHM, Pieterse CMJ, van Loon LC. (2007) Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 97: 239–243 - PubMed
-
- Benning C. (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. In Annual Review of Cell and Developmental Biology, Vol 25 Annual Reviews, Palo Alto, CA, pp 71–91 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

