Recovery of otoacoustic emissions after high-level noise exposure in the American bullfrog
- PMID: 24501139
- PMCID: PMC4006586
- DOI: 10.1242/jeb.090092
Recovery of otoacoustic emissions after high-level noise exposure in the American bullfrog
Erratum in
- J Exp Biol. 2014 Jun 15;217(Pt 12):2221
Abstract
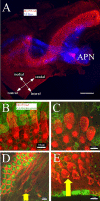
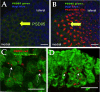
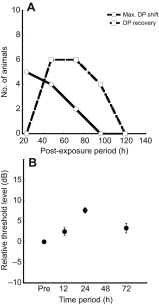
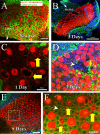
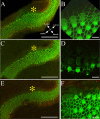
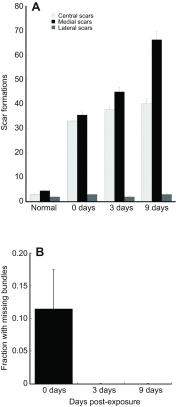
The American bullfrog (Rana catesbeiana) has an amphibian papilla (AP) that senses airborne, low-frequency sound and generates distortion product otoacoustic emissions (DPOAEs) similar to other vertebrate species. Although ranid frogs are typically found in noisy environments, the effects of noise on the AP have not been studied. First, we determined the noise levels that diminished DPOAE at 2f1-f2 using an f2 stimulus level at 80 dB SPL and that also produced morphological damage of the sensory epithelium. Second, we compared DPOAE (2f1-f2) responses with histopathologic changes occurring in bullfrogs after noise exposure. Consistent morphological damage, such as fragmented hair cells and missing bundles, as well as elimination of DPOAE responses were seen only after very high-level (>150 dB SPL) sound exposures. The morphological response of hair cells to noise differed along the mediolateral AP axis: medial hair cells were sensitive to noise and lateral hair cells were relatively insensitive to noise. Renewed or repaired hair cells were not observed until 9 days post-exposure. Following noise exposure, DPOAE responses disappeared within 24 h and then recovered to normal pre-exposure levels within 3-4 days. Our results suggest that DPOAEs in the bullfrog are sensitive to the initial period of hair cell damage. After noise-induced damage, the bullfrog AP has functional recovery mechanisms that do not depend on substantial hair cell regeneration or repair. Thus, the bullfrog auditory system might serve as an interesting model for investigation of ways to prevent noise damage.
Keywords: Active amplification; Cubic distortion product; Hair Cells; Hearing loss; Regeneration.
Figures


References
-
- Baker R. J., Wilson J. P., Whitehead M. L. (1989). Otoacoustic evidence for nonlinear behavior in frog hearing: suppression but no distortion products. In Cochlear Mechanisms: Structure, Function and Models (ed. Wilson J., Kemp D. T.), pp. 349-356 New York, NY: Plenum;
-
- Bao J., Lin H., Ouyang Y., Lei D., Osman A., Kim T.-W., Mei L., Dai P., Ohlemiller K. K., Ambron R. T. (2004). Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat. Neurosci. 7, 1250-1258 - PubMed
-
- Brown D. K., Bowman D. M., Kimberley B. P. (2000). The effects of maturation and stimulus parameters on the optimal f(2)/f(1) ratio of the 2f(1)-f(2) distortion product otoacoustic emission in neonates(1). Hear. Res. 145, 17-24 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

