Tamoxifen-DNA adduct formation in monkey and human reproductive organs
- PMID: 24501327
- PMCID: PMC4004208
- DOI: 10.1093/carcin/bgu029
Tamoxifen-DNA adduct formation in monkey and human reproductive organs
Abstract
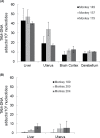
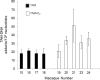
The estrogen analog tamoxifen (TAM), used for adjuvant therapy of breast cancer, induces endometrial and uterine tumors in breast cancer patients. Proliferation stimulus of the uterine endometrium is likely involved in tumor induction, but genotoxicity may also play a role. Formation of TAM-DNA adducts in human tissues has been reported but remains controversial. To address this issue, we examined TAM-DNA adducts in uteri from two species of monkeys, Erythrocebus patas (patas) and Macaca fascicularis (macaque), and in human endometrium and myometrium. Monkeys were given 3-4 months of chronic TAM dosing scaled to be equivalent to the daily human dose. In the uteri, livers and brains from the patas (n = 3), and endometrium from the macaques (n = 4), TAM-DNA adducts were measurable by TAM-DNA chemiluminescence immunoassay. Average TAM-DNA adduct values for the patas uteri (23 adducts/10(8) nucleotides) were similar to those found in endometrium of the macaques (19 adducts/10(8) nucleotides). Endometrium of macaques exposed to both TAM and low-dose estradiol (n = 5) averaged 34 adducts/10(8) nucleotides. To examine TAM-DNA persistence in the patas, females (n = 3) were exposed to TAM for 3 months and to no drug for an additional month, resulting in low or non-detectable TAM-DNA in livers and uteri. Human endometrial and myometrial samples from women receiving (n = 8) and not receiving (n = 8) TAM therapy were also evaluated. Women receiving TAM therapy averaged 10.3 TAM-DNA adducts/10(8) nucleotides, whereas unexposed women showed no detectable TAM-DNA. The data indicate that genotoxicity, in addition to estrogen agonist effects, may contribute to TAM-induced human endometrial cancer.
Figures
References
-
- Early Breast Cancer Trialists’ Collaborative Group (1998). Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet, 351, 1451–1467 - PubMed
-
- Fisher B., et al. (1998). Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst., 90, 1371–1388 - PubMed
-
- IARC (2012). A review of human carcinogens - tamoxifen. IARC Monographs on the Evaluation of Carcinogenic Risks to Human, Vol. 100A, 131–162 International Agency for Research on Cancer, Lyon
-
- Twombly R. (2002). FDA issues warning about ‘new’ tamoxifen risk. J. Natl Cancer Inst., 94, 1122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

