The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders
- PMID: 24501344
- PMCID: PMC6608531
- DOI: 10.1523/JNEUROSCI.4131-13.2014
The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders
Abstract
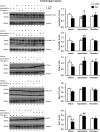
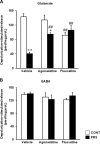
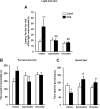
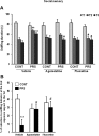
Abnormalities of synaptic transmission in the hippocampus represent an integral part of the altered programming triggered by early life stress, which enhances the vulnerability to stress-related disorders in the adult life. Rats exposed to prenatal restraint stress (PRS) develop enduring biochemical and behavioral changes characteristic of an anxious/depressive-like phenotype. Most neurochemical abnormalities in PRS rats are found in the ventral hippocampus, a region that encodes memories related to stress and emotions. We have recently demonstrated a causal link between the reduction of glutamate release in the ventral hippocampus and anxiety-like behavior in PRS rats. To confer pharmacological validity to the glutamatergic hypothesis of stress-related disorders, we examined whether chronic treatment with two antidepressants with different mechanisms of action could correct the defect in glutamate release and associated behavioral abnormalities in PRS rats. Adult unstressed or PRS rats were treated daily with either agomelatine (40 mg/kg, i.p.) or fluoxetine (5 mg/kg, i.p.) for 21 d. Both treatments reversed the reduction in depolarization-evoked glutamate release and in the expression of synaptic vesicle-associated proteins in the ventral hippocampus of PRS rats. Antidepressant treatment also corrected abnormalities in anxiety-/depression-like behavior and social memory performance in PRS rats. The effect on glutamate release was strongly correlated with the improvement of anxiety-like behavior and social memory. These data offer the pharmacological demonstration that glutamatergic hypofunction in the ventral hippocampus lies at the core of the pathological phenotype caused by early life stress and represents an attractive pharmacological target for novel therapeutic strategies.
Figures
Similar articles
-
Anxiety-like behavior of prenatally stressed rats is associated with a selective reduction of glutamate release in the ventral hippocampus.J Neurosci. 2012 Nov 28;32(48):17143-54. doi: 10.1523/JNEUROSCI.1040-12.2012. J Neurosci. 2012. PMID: 23197707 Free PMC article.
-
Activation of presynaptic oxytocin receptors enhances glutamate release in the ventral hippocampus of prenatally restraint stressed rats.Psychoneuroendocrinology. 2015 Dec;62:36-46. doi: 10.1016/j.psyneuen.2015.07.005. Epub 2015 Jul 18. Psychoneuroendocrinology. 2015. PMID: 26231445
-
Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats.Psychopharmacology (Berl). 2011 Oct;217(3):301-13. doi: 10.1007/s00213-011-2280-x. Epub 2011 Apr 19. Psychopharmacology (Berl). 2011. PMID: 21503609
-
Epigenetic programming of the stress response in male and female rats by prenatal restraint stress.Brain Res Rev. 2008 Mar;57(2):571-85. doi: 10.1016/j.brainresrev.2007.11.004. Epub 2007 Nov 28. Brain Res Rev. 2008. PMID: 18164765 Review.
-
Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems.Stress. 2007 Aug;10(3):227-49. doi: 10.1080/10253890701223130. Stress. 2007. PMID: 17613938 Review.
Cited by
-
Agomelatine for the treatment of generalized anxiety disorder: focus on its distinctive mechanism of action.Ther Adv Psychopharmacol. 2022 Jun 30;12:20451253221105128. doi: 10.1177/20451253221105128. eCollection 2022. Ther Adv Psychopharmacol. 2022. PMID: 35795687 Free PMC article. Review.
-
Gut microbiota-mediated metabolic restructuring aggravates emotional deficits after anesthesia/surgery in rats with preoperative stress.Front Immunol. 2022 Aug 8;13:819289. doi: 10.3389/fimmu.2022.819289. eCollection 2022. Front Immunol. 2022. PMID: 36003406 Free PMC article.
-
Epigenetic landscape of stress surfeit disorders: Key role for DNA methylation dynamics.Int Rev Neurobiol. 2021;156:127-183. doi: 10.1016/bs.irn.2020.08.002. Epub 2020 Sep 30. Int Rev Neurobiol. 2021. PMID: 33461662 Free PMC article.
-
Behavioral Animal Models and Neural-Circuit Framework of Depressive Disorder.Neurosci Bull. 2025 Feb;41(2):272-288. doi: 10.1007/s12264-024-01270-7. Epub 2024 Aug 9. Neurosci Bull. 2025. PMID: 39120643 Free PMC article. Review.
-
Lasting Differential Effects on Plasticity Induced by Prenatal Stress in Dorsal and Ventral Hippocampus.Neural Plast. 2016;2016:2540462. doi: 10.1155/2016/2540462. Epub 2016 Jan 10. Neural Plast. 2016. PMID: 26881096 Free PMC article. Review.
References
-
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16:634–646. doi: 10.1038/mp.2010.44. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
