A map of functional synaptic connectivity in the mouse anteroventral cochlear nucleus
- PMID: 24501361
- PMCID: PMC3913869
- DOI: 10.1523/JNEUROSCI.4669-13.2014
A map of functional synaptic connectivity in the mouse anteroventral cochlear nucleus
Abstract
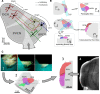
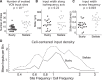
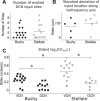
The cochlear nuclei are the first central processors of auditory information and provide inputs to all the major brainstem and midbrain auditory nuclei. Although the local circuits within the cochlear nuclei are understood at a cellular level, the spatial patterns of connectivity and the connection strengths in these circuits have been less well characterized. We have applied a novel, quantitative approach to mapping local circuits projecting to cells in the mouse anteroventral cochlear nucleus (AVCN) using laser-scanning photostimulation and glutamate uncaging. The amplitude and kinetics of individual evoked synaptic events were measured to reveal the patterns and strengths of synaptic connections. We found that the two major excitatory projection cell classes, the bushy and T-stellate cells, receive a spatially broad inhibition from D-stellate cells in the AVCN, and a spatially confined inhibition from the tuberculoventral cells of the dorsal cochlear nucleus. Furthermore, T-stellate cells integrate D-stellate inhibition from an area that spans twice the frequency range of that integrated by bushy cells. A subset of both bushy and T-stellate cells receives inhibition from an unidentified cell population at the dorsal-medial boundary of the AVCN. A smaller subset of cells receives local excitation from within the AVCN. Our results show that inhibitory circuits can have target-specific patterns of spatial convergence, synaptic strength, and receptor kinetics, resulting in different spectral and temporal processing capabilities.
Keywords: brain mapping; glutamate uncaging; inhibitory function; laser photostimulation; local circuits; topography.
Figures








References
-
- Blackburn CC, Sachs MB. Classification of unit types in the anteroventral cochlear nucleus: PST histograms and regularity analysis. J Neurophysiol. 1989;62:1303–1329. - PubMed
-
- Blackburn CC, Sachs MB. The representations of the steady-state vowel sound /e/ in the discharge patterns of cat anteroventral cochlear nucleus neurons. J Neurophysiol. 1990;63:1191–1212. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
