Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain
- PMID: 24501367
- PMCID: PMC3913872
- DOI: 10.1523/JNEUROSCI.4037-13.2014
Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain
Abstract
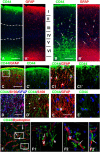
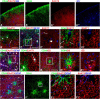
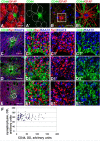
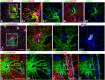
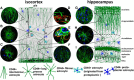
To examine the diversity of astrocytes in the human brain, we immunostained surgical specimens of temporal cortex and hippocampus and autopsy brains for CD44, a plasma membrane protein and extracellular matrix receptor. CD44 antibodies outline the details of astrocyte morphology to a degree not possible with glial fibrillary acidic protein (GFAP) antibodies. CD44+ astrocytes could be subdivided into two groups. First, CD44+ astrocytes with long processes were consistently found in the subpial area ("interlaminar" astrocytes), the deep isocortical layers, and the hippocampus. Many of these processes ended on blood vessels. Some were also found adjacent to large blood vessels, from which they extended long processes. We observed these CD44+, long-process astrocytes in every brain we examined, from fetal to adult. These astrocytes generally displayed high immunostaining for GFAP, S100β, and CD44, but low immunostaining for glutamine synthetase, excitatory amino-acid transporter 1 (EAAT1), and EAAT2. Aquaporin 4 (AQP4) appeared distributed all over the cell bodies and processes of the CD44+ astrocytes, while, in contrast, AQP4 localized to perivascular end feet in the CD44- protoplasmic astrocytes. Second, there were CD44+ astrocytes without long processes in the cortex. These were not present during gestation or at birth, and in adult brains varied substantially in number, shape, and immunohistochemical phenotype. Many of these displayed a "mixed" morphological and immunocytochemical phenotype between protoplasmic and fibrous astrocytes. We conclude that the diversity of astrocyte populations in the isocortex and archicortex in the human brain reflects both intrinsic and acquired phenotypes, the latter perhaps representing a shift from CD44- "protoplasmic" to CD44+ "fibrous"-like astrocytes.
Keywords: CD44; astrocyte; interlaminar astrocyte; protoplasmic astrocyte.
Figures







References
-
- Bignami A, Dahl D. Brain-specific hyaluronate-binding protein. A product of white matter astrocytes? J Neurocytol. 1986;15:671–679. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
