BAF-1 mobility is regulated by environmental stresses
- PMID: 24501420
- PMCID: PMC3967975
- DOI: 10.1091/mbc.E13-08-0477
BAF-1 mobility is regulated by environmental stresses
Abstract
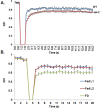
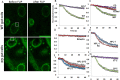
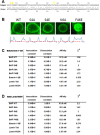
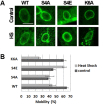
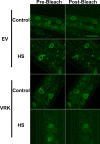
Barrier to autointegration factor (BAF) is an essential component of the nuclear lamina that binds lamins, LEM-domain proteins, histones, and DNA. Under normal conditions, BAF protein is highly mobile when assayed by fluorescence recovery after photobleaching and fluorescence loss in photobleaching. We report that Caenorhabditis elegans BAF-1 mobility is regulated by caloric restriction, food deprivation, and heat shock. This was not a general response of chromatin-associated proteins, as food deprivation did not affect the mobility of heterochromatin protein HPL-1 or HPL-2. Heat shock also increased the level of BAF-1 Ser-4 phosphorylation. By using missense mutations that affect BAF-1 binding to different partners we find that, overall, the ability of BAF-1 mutants to be immobilized by heat shock in intestinal cells correlated with normal or increased affinity for emerin in vitro. These results show BAF-1 localization and mobility at the nuclear lamina are regulated by stress and unexpectedly reveal BAF-1 immobilization as a specific response to caloric restriction in C. elegans intestinal cells.
Figures

References
-
- Asencio C, Davidson IF, Santarella-Mellwig R, Ly-Hartig TB, Mall M, Wallenfang MR, Mattaj IW, Gorjánácz M. Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell. 2012;150:122–135. - PubMed
-
- Ben-Harush K, Wiesel N, Frenkiel-Krispin D, Moeller D, Soreq E, Aebi U, Herrmann H, Gruenbaum Y, Medalia O. The supramolecular organization of the C. elegans nuclear lamin filament. J Mol Biol. 2009;386:1392–1402. - PubMed
-
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

