Optimization of the lactam side chain of 7-azaindenoisoquinoline topoisomerase I inhibitors and mechanism of action studies in cancer cells
- PMID: 24502276
- PMCID: PMC3983387
- DOI: 10.1021/jm401471v
Optimization of the lactam side chain of 7-azaindenoisoquinoline topoisomerase I inhibitors and mechanism of action studies in cancer cells
Abstract
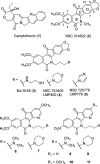
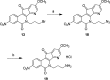
Optimization of the lactam ω-aminoalkyl substituents in a series of 7-azaindenoisoquinolines resulted in new anticancer agents with improved Top1 inhibitory potencies and cancer cell cytotoxicities. The new compounds 14-17 and 19 exhibited mean graph midpoint cytotoxicity (GI50) values of 21-71 nM in the NCI panel of 60 human cancer cell cultures. Ternary 7-azaindenoisoquinoline-DNA-Top1 cleavage complexes that persist for up to 6 h were detected in HCT116 colon cancer cells. Ternary complexes containing 7-azaindenoisoquinolines were significantly more stable than those in which camptothecin was incorporated. DNA content distribution histograms showed S-phase block 3 h after drug removal. Drug-induced DNA damage in HCT116 cells was revealed by induction of the histone γ-H2AX marker. The 7-azaindenoisoquinolines were able to partially overcome resistance in several drug-resistant cell lines, and they were not substrates for the ABCB1 drug efflux transporter. Molecular modeling studies indicate that the 7-azaindenoisoquinolines intercalate at the DNA cleavage site in DNA-Top1 covalent complexes with the lactam side chain projecting into the major groove. Overall, the results indicate that the 7-azaindenoisoquinolines are promising anticancer agents that merit further development.
Figures







References
-
- Wall M. E.; Wani M. C.; Cook C. E.; Palmer K. H.; McPhail A. T.; Sim G. A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890.
-
- Kohlhagen G.; Paull K.; Cushman M.; Nagafuji P.; Pommier Y. Protein-Linked DNA Strand Breaks Induced by NSC 314622, a Novel Noncamptothecin Topoisomerase I Poison. Mol. Pharmacol. 1998, 54, 50–58. - PubMed
-
- Khadka D. B.; Cho W.-J. Topoisomerase Inhibitors as Anticancer Agents: A Patent Update. Expert Opin. Ther. Pat. 2013, 23, 1033–1056. - PubMed
-
- Staker B. L.; Feese M. D.; Cushman M.; Pommier Y.; Zembower D.; Stewart L.; Burgin A. B. Structures of Three Classes of Anticancer Agents Bound to the Human Topoisomerase I-DNA Covalent Complex. J. Med. Chem. 2005, 48, 2336–2345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

