Diazirine-containing photoactivatable isoprenoid: synthesis and application in studies with isoprenylcysteine carboxyl methyltransferase
- PMID: 24502619
- PMCID: PMC3985442
- DOI: 10.1021/jo402600b
Diazirine-containing photoactivatable isoprenoid: synthesis and application in studies with isoprenylcysteine carboxyl methyltransferase
Abstract
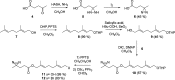
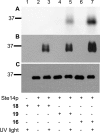
Photoaffinity labeling is a useful technique employed to identify protein-ligand and protein-protein noncovalent interactions. Photolabeling experiments have been particularly informative for probing membrane-bound proteins where structural information is difficult to obtain. The most widely used classes of photoactive functionalities include aryl azides, diazocarbonyls, diazirines, and benzophenones. Diazirines are intrinsically smaller than benzophenones and generate carbenes upon photolysis that react with a broader range of amino acid side chains compared with the benzophenone-derived diradical; this makes diazirines potentially more general photoaffinity-labeling agents. In this article, we describe the development and application of a new isoprenoid analogue containing a diazirine moiety that was prepared in six steps and incorporated into an a-factor-derived peptide produced via solid-phase synthesis. In addition to the diazirine moiety, fluorescein and biotin groups were also incorporated into the peptide to aid in the detection and enrichment of photo-cross-linked products. This multifuctional diazirine-containing peptide was a substrate for Ste14p, the yeast homologue of the potential anticancer target Icmt, with K(m) (6.6 μM) and V(max) (947 pmol min(-1) mg(-1)) values comparable or better than a-factor peptides functionalized with benzophenone-based isoprenoids. Photo-cross-linking experiments demonstrated that the diazirine probe photo-cross-linked to Ste14p with observably higher efficiency than benzophenone-containing a-factor peptides.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

