Species and gender differences in the carcinogenic activity of trimethylolpropane triacrylate in rats and mice
- PMID: 24503412
- PMCID: PMC3995139
- DOI: 10.1016/j.fct.2014.01.048
Species and gender differences in the carcinogenic activity of trimethylolpropane triacrylate in rats and mice
Abstract
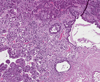
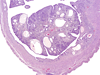
Trimethylolpropane triacrylate (TMPTA) is a multifunctional monomer with industrial applications. To determine the carcinogenic potential, male and female F344/N rats and B6C3F1/N mice were administered TMPTA (0, 0.3, 1.0, or 3.0mg/kg) in acetone dermally for 2 years. There were no differences in the body weights and survival in the treated animals compared to controls. Nonneoplastic skin lesions at the site of application included epidermal hyperplasia and hyperkeratosis in both rats and mice. There were no incidences of tumors at the site of application in rats and mice. Rare malignant liver neoplasms were observed in female mice that included hepatoblastoma in the 0.3 and 3.0mg/kg groups, and hepatocholangiocarcinoma in the 1.0 and 3.0mg/kg groups. The incidences of uterine stromal polyp and stromal polyp or stromal sarcoma (combined) in female mice occurred with positive trends and the incidences were significantly increased in the 3.0mg/kg group. A marginal increase in the incidences of malignant mesothelioma in male rats may have been related to TMPTA treatment. In conclusion, our studies show that TMPTA is a dermal irritant in both rats and mice of either sex. Increased incidences of tumor formation were observed in female mice and male rats.
Keywords: Carcinogenicity; Liver; Skin; Trimethylolpropane triacrylate; Uterine.
Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Adams ET, Auerbach S, Blackshear PE, Bradley A, Gruebbel MM, Little PB, Malarkey D, Maronpot R, McKay JS, Miller RA, Moore RR, Morrison JP, Nyska A, Ramot Y, Rao D, Suttie A, Wells MY, Willson GA, Elmore SA. Proceedings of the 2010 national toxicology program satellite symposium. Toxicol. Pathol. 2011;39:240–266. - PMC - PubMed
-
- Andrews LS, Clary JJ. Review of the toxicity of multifunctional acrylates. J. Toxicol. Environ. Health. 1986;19:149–164. - PubMed
-
- Attanoos RL, Gibbs AR. Pathology of malignant mesothelioma. Histopathology. 1997;30:403–418. - PubMed
-
- Bach U, Hailey JR, Hill GD, Kaufmann W, Latimer KS, Malarkey DE, Maronpot RM, Miller RA, Moore RR, Morrison JP, Nolte T, Rinke M, Rittinghausen S, Suttie AW, Travlos GS, Vahle JL, Willson GA, Elmore SA. Proceedings of the 2009 national toxicology program satellite symposium. Toxicol. Pathol. 2010;38:9–36. - PMC - PubMed
-
- Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induced mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–431. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

