Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer
- PMID: 24503539
- PMCID: PMC3944233
- DOI: 10.1038/cddis.2014.14
Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer
Abstract
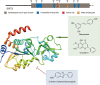
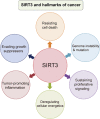
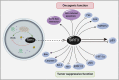
Sirtuin-3 (SIRT3), a major mitochondria NAD+-dependent deacetylase, may target mitochondrial proteins for lysine deacetylation and also regulate cellular functions. And, SIRT3 is an emerging instrumental regulator of the mitochondrial adaptive response to stress, such as metabolic reprogramming and antioxidant defense mechanisms. Accumulating evidence has recently demonstrated that SIRT3 may function as either oncogene or tumor suppressor on influencing cell death by targeting a series of key modulators and their relevant pathways in cancer. Thus, in this review, we present the structure, transcriptional regulation, and posttranslational modifications of SIRT3. Subsequently, we focus on highlighting the Janus role of SIRT3 with oncogenic or tumor-suppressive function in cancer, which may provide more new clues for exploring SIRT3 as a therapeutic target for drug discovery.
Figures

References
-
- Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mito- chondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

