Use of monitoring levels of soluble forms of cytokeratin 18 in the urine of patients with superficial bladder cancer following intravesical Ad-IFNα/Syn3 treatment in a phase l study
- PMID: 24503570
- PMCID: PMC3962717
- DOI: 10.1038/cgt.2014.1
Use of monitoring levels of soluble forms of cytokeratin 18 in the urine of patients with superficial bladder cancer following intravesical Ad-IFNα/Syn3 treatment in a phase l study
Abstract
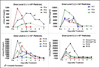
A phase l study using intravesical Ad-IFNαSyn3 for patients with bacillus Calmette-Guérin-resistant superficial bladder cancer showed a complete remission (CR) of 43% at 90 days after treatment with high levels of interferon-α (IFNα) being produced. Ad-IFNα kills bladder cancer cells by two apoptotic and one necrotic mechanism that can be measured by soluble forms of cytokeratin 18 (CK18) using M30 and M65 ELISAs, assays for caspase-cleaved (apoptotic) and uncleaved (necrotic) cell death, respectively. Therefore, we determined whether M30 and M65 levels in the urine after treatment could document all three mechanisms of cancer cell kill and also predict having a CR. High levels of both M30 and M65 were found in all patients within 24 h after treatment with all three types of cancer cell death occurring. Moreover, the return of both M30 and M65 levels in the urine to normal levels within 5 days or more after treatment was strongly associated with obtaining a CR (P=0.003). This is the first time that such assays have been used to study response to therapy in the urine of patients with bladder cancer and in the future may prove valuable in predicting clinical outcome.
Figures
References
-
- Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, et al. Intravesical Ad-IFN alpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-alpha protein. Mol Ther. 2004;10(3):525–532. - PubMed
-
- Tao Z, Connor RJ, Ashoori F, Dinney CP, Munsell M, Philopena JA, Benedict WF. Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained:implications for clinical investigation. Cancer Gene Therapy. 2006;13(2):125–130. - PubMed
-
- Papageorgiou A, Lashinger L, Millikan R, Grossman HB, Benedict WF, Dinney CP, McConkey DJ. Role of tumor necrosis factor-related apoptosis-inducing ligand in interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2004;64(24):8973–8979. - PubMed
-
- Zhang X-Q, Yang Z, Dong L, Papageorgiou A, McConkey D, Benedict WF. Adenoviral- mediated interferon α overcomes resistance to the interferon protein in various cancer types and has marked bystander effects. Cancer Gene Therapy. 2007;14:241–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

