Vaccination of mice using the West Nile virus E-protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects mice against a lethal challenge
- PMID: 24503579
- PMCID: PMC3913677
- DOI: 10.1371/journal.pone.0087837
Vaccination of mice using the West Nile virus E-protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects mice against a lethal challenge
Erratum in
- PLoS One. 2014;9(2):e91631
Abstract
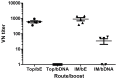
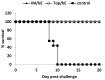
West Nile virus (WNV) is a mosquito-borne flavivirus that is endemic in Africa, the Middle East, Europe and the United States. There is currently no antiviral treatment or human vaccine available to treat or prevent WNV infection. DNA plasmid-based vaccines represent a new approach for controlling infectious diseases. In rodents, DNA vaccines have been shown to induce B cell and cytotoxic T cell responses and protect against a wide range of infections. In this study, we formulated a plasmid DNA vector expressing the ectodomain of the E-protein of WNV into nanoparticles by using linear polyethyleneimine (lPEI) covalently bound to mannose and examined the potential of this vaccine to protect against lethal WNV infection in mice. Mice were immunized twice (prime--boost regime) with the WNV DNA vaccine formulated with lPEI-mannose using different administration routes (intramuscular, intradermal and topical). In parallel a heterologous boost with purified recombinant WNV envelope (E) protein was evaluated. While no significant E-protein specific humoral response was generated after DNA immunization, protein boosting of DNA-primed mice resulted in a marked increase in total neutralizing antibody titer. In addition, E-specific IL-4 T-cell immune responses were detected by ELISPOT after protein boost and CD8(+) specific IFN-γ expression was observed by flow cytometry. Challenge experiments using the heterologous immunization regime revealed protective immunity to homologous and virulent WNV infection.
Conflict of interest statement
Figures




References
-
- Hayes EB, Gubler DJ (2006) West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med 57: 181–194. - PubMed
-
- Watson JT, Pertel PE, Jones RC, Siston AM, Paul WS, et al. (2004) Clinical characteristics and functional outcomes of West Nile Fever. Ann Intern Med 141: 360–365. - PubMed
-
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, et al. (1999) Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286: 2333–2337. - PubMed
-
- Gubler DJ (2007) The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis 45: 1039–1046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

