Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer's disease
- PMID: 24503708
- PMCID: PMC3909893
- DOI: 10.1038/emm.2013.147
Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer's disease
Abstract
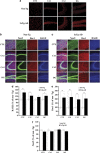
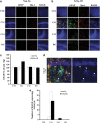
The receptor for advanced glycation end products (RAGE) has been reported to have a pivotal role in the pathogenesis of Alzheimer's disease (AD). This study investigated RAGE levels in the hippocampus and cortex of a triple transgenic mouse model of AD (3xTg-AD) using western blotting and immunohistochemical double-labeling to assess cellular localization. Analysis of western blots showed that there were no differences in the hippocampal and cortical RAGE levels in 10-month-old adult 3xTg-AD mice, but significant increases in RAGE expression were found in the 22- to 24-month-old aged 3xTg-AD mice compared with those of age-matched controls. RAGE-positive immunoreactivity was observed primarily in neurons of aged 3xTg-AD mice with very little labeling in non-neuronal cells, with the notable exception of RAGE presence in astrocytes in the hippocampal area CA1. In addition, RAGE signals were co-localized with the intracellular amyloid precursor protein (APP)/amyloid beta (Aβ) but not with the extracellular APP/Aβ. In aged 3xTg-AD mice, expression of human tau was observed in the hippocampal area CA1 and co-localized with RAGE signals. The increased presence of RAGE in the 3xTg-AD animal model showing critical aspects of AD neuropathology indicates that RAGE may contribute to cellular dysfunction in the AD brain.
Figures



References
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physioll Rev. 2001;81:741–766. - PubMed
-
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
-
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. - PubMed
-
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24:1063–1070. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
