Choose your label wisely: water-soluble fluorophores often interact with lipid bilayers
- PMID: 24503716
- PMCID: PMC3913624
- DOI: 10.1371/journal.pone.0087649
Choose your label wisely: water-soluble fluorophores often interact with lipid bilayers
Abstract
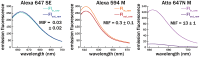
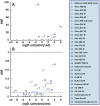
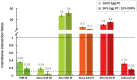
Water-soluble organic fluorophores are widely used as labels in biological systems. However, in many cases these fluorophores can interact strongly with lipid bilayers, influencing the interaction of the target with the bilayer and/or leading to misleading fluorescent signals. Here, we quantify the interaction of 32 common water-soluble dyes with model lipid bilayers to serve as an additional criterion when selecting a dye label.
Conflict of interest statement
Figures


References
-
- Loidl-Stahlhofen A, Eckert A, Hartmann T, Schöttner MJ (2001) Solid-supported lipid membranes as a tool for determination of membrane affinity: High-throughput screening of a physicochemical parameter. Pharm. Sci. 90: 599–606. - PubMed
-
- Pauletti GM, Wunderli-Allenspach H (1994) Partition coefficients in vitro: artificial membranes as a standardized distribution model. Eur. J. Pharm. Sci. 1: 273–282.
-
- White SH, Wimley WC, Ladokhin AS, Hristova K (1998) [4] Protein folding in membranes: Determining energetics of peptide-bilayer interactions. Methods Enzymol. 295: 62–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

