Evaluating retinopathy of prematurity screening guidelines for 24- to 27-week gestational age infants
- PMID: 24503911
- PMCID: PMC3969774
- DOI: 10.1038/jp.2014.12
Evaluating retinopathy of prematurity screening guidelines for 24- to 27-week gestational age infants
Abstract
Objective: To determine whether current retinopathy of prematurity (ROP) screening guidelines adequately identify treatable ROP in a contemporary cohort of extremely low gestation infants.
Study design: Data from the Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial were used. Inborn infants of 24 (0)/7 to 27 (6)/7 weeks gestational age (GA) with consent before delivery were enrolled in 2005 to 2009. Severe ROP (type 1 ROP or treatment with laser, cryotherapy or bevacizumab) or death was the primary outcome for the randomized trial. Examinations followed the then current AAP (American Academy of Pediatrics) screening recommendations, beginning by 31 to 33 weeks postmenstrual age (PMA).
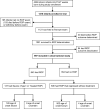
Result: One thousand three hundred and sixteen infants were enrolled in the trial. Nine hundred and ninety-seven of the 1121 who survived to first eye exam had final ROP outcome determined. One hundred and thirty-seven (14% of 997) met criteria for severe ROP and 128 (93%) of those had sufficient data (without missing or delayed exams) to determine age of onset of severe ROP. PMA at onset was 32.1 to 53.1 weeks. In this referral center cohort, 1.4% (14/997) developed severe ROP after discharge.
Conclusion: Our contemporary data support the 2013 AAP screening guidelines for ROP for infants of 24 (0)/7 to 27 (6)/7 weeks GA. Some infants do not meet treatment criteria until after discharge home. Post-discharge follow-up of infants who are still at risk for severe ROP is crucial for timely detection and treatment.
Figures



References
-
- American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, and American Association of Certified Orthoptists Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–195. - PubMed
-
- American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Ophthalmology Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2001;108:809–811. - PubMed
-
- American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–576. - PubMed
-
- Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB, et al. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98:1628–1640. - PubMed
Publication types
MeSH terms
Grants and funding
- U10 HD021364/HD/NICHD NIH HHS/United States
- UG1 HD087229/HD/NICHD NIH HHS/United States
- UG1 HD053089/HD/NICHD NIH HHS/United States
- U10 HD040461/HD/NICHD NIH HHS/United States
- U10 HD040689/HD/NICHD NIH HHS/United States
- U10 HD027904/HD/NICHD NIH HHS/United States
- UG1 HD034216/HD/NICHD NIH HHS/United States
- UG1 HD027880/HD/NICHD NIH HHS/United States
- U10 HD034216/HD/NICHD NIH HHS/United States
- UL1 TR000371/TR/NCATS NIH HHS/United States
- U10 HD027856/HD/NICHD NIH HHS/United States
- U10 HD021373/HD/NICHD NIH HHS/United States
- U10 HD027880/HD/NICHD NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

