Antiinflammatory therapy outcomes for mild OSA in children
- PMID: 24504096
- PMCID: PMC4077412
- DOI: 10.1378/chest.13-2288
Antiinflammatory therapy outcomes for mild OSA in children
Abstract
Background: OSA is highly prevalent in children and usually initially treated by adenotonsillectomy. Nonsurgical alternatives for mild OSA primarily consisting of antiinflammatory approaches have emerged, but their efficacy has not been extensively assessed.
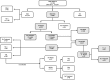
Methods: A retrospective review of clinically and polysomnographically diagnosed patients with OSA treated between 2007 and 2012 was performed to identify otherwise healthy children ages 2 to 14 years who fulfilled the criteria for mild OSA and who were treated with a combination of intranasal corticosteroid and oral montelukast (OM) for 12 weeks (ICS + OM). A subset of children continued OM treatment for 6 to 12 months.
Results: A total of 3,071 children were diagnosed with OSA, of whom 836 fulfilled mild OSA criteria and 752 received ICS + OM. Overall, beneficial effects occurred in > 80% of the children, with nonadherence being documented in 61 children and adenotonsillectomy being ultimately performed in 12.3%. Follow-up polysomnography in a subset of 445 patients showed normalization of sleep findings in 62%, while 17.1% showed either no improvement or worsening of their OSA. Among the latter, older children (aged > 7 years; OR, 2.3; 95% CI, 1.43-4.13; P < .001) and obese children (BMI z-score > 1.65; OR: 6.3; 95% CI, 4.23-11.18; P < .000001) were significantly more likely to be nonresponders.
Conclusions: A combination of ICS + OM as initial treatment of mild OSA appears to provide an effective alternative to adenotonsillectomy, particularly in younger and nonobese children. These results support implementation of multicenter randomized trials to more definitively establish the role of ICS + OM treatment in pediatric OSA.
Figures
Comment in
-
Repeated polysomnograms after antiinflammatory therapy of mild pediatric OSA.Chest. 2014 Dec;146(6):e226. doi: 10.1378/chest.14-1988. Chest. 2014. PMID: 25451371 No abstract available.
-
Response.Chest. 2014 Dec;146(6):e226-7. doi: 10.1378/chest.14-2043. Chest. 2014. PMID: 25451372 Free PMC article. No abstract available.
References
-
- Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714-e755 - PubMed
-
- Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676-683 - PubMed
-
- Kheirandish-Gozal L, Kim J, Goldbart AD, Gozal D. Novel pharmacological approaches for treatment of obstructive sleep apnea in children. Expert Opin Investig Drugs. 2013;22(1):71-85 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical