Detection of cancer DNA in plasma of patients with early-stage breast cancer
- PMID: 24504125
- PMCID: PMC4024333
- DOI: 10.1158/1078-0432.CCR-13-2933
Detection of cancer DNA in plasma of patients with early-stage breast cancer
Abstract
Purpose: Detecting circulating plasma tumor DNA (ptDNA) in patients with early-stage cancer has the potential to change how oncologists recommend systemic therapies for solid tumors after surgery. Droplet digital polymerase chain reaction (ddPCR) is a novel sensitive and specific platform for mutation detection.
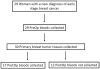
Experimental design: In this prospective study, primary breast tumors and matched pre- and postsurgery blood samples were collected from patients with early-stage breast cancer (n = 29). Tumors (n = 30) were analyzed by Sanger sequencing for common PIK3CA mutations, and DNA from these tumors and matched plasma were then analyzed for PIK3CA mutations using ddPCR.
Results: Sequencing of tumors identified seven PIK3CA exon 20 mutations (H1047R) and three exon 9 mutations (E545K). Analysis of tumors by ddPCR confirmed these mutations and identified five additional mutations. Presurgery plasma samples (n = 29) were then analyzed for PIK3CA mutations using ddPCR. Of the 15 PIK3CA mutations detected in tumors by ddPCR, 14 of the corresponding mutations were detected in presurgical ptDNA, whereas no mutations were found in plasma from patients with PIK3CA wild-type tumors (sensitivity 93.3%, specificity 100%). Ten patients with mutation-positive ptDNA presurgery had ddPCR analysis of postsurgery plasma, with five patients having detectable ptDNA postsurgery.
Conclusions: This prospective study demonstrates accurate mutation detection in tumor tissues using ddPCR, and that ptDNA can be detected in blood before and after surgery in patients with early-stage breast cancer. Future studies can now address whether ptDNA detected after surgery identifies patients at risk for recurrence, which could guide chemotherapy decisions for individual patients.
©2014 American Association for Cancer Research.
Figures
Comment in
-
Minimal residual disease in breast cancer: in blood veritas.Clin Cancer Res. 2014 May 15;20(10):2505-7. doi: 10.1158/1078-0432.CCR-14-0370. Epub 2014 Mar 21. Clin Cancer Res. 2014. PMID: 24658155
-
Detecting Plasma Tumor DNA in Early-Stage Breast Cancer-Letter.Clin Cancer Res. 2015 Aug 1;21(15):3569. doi: 10.1158/1078-0432.CCR-15-0776. Clin Cancer Res. 2015. PMID: 26240292 No abstract available.
-
Detecting Plasma Tumor DNA in Early-Stage Breast Cancer--Reply.Clin Cancer Res. 2015 Aug 1;21(15):3570. doi: 10.1158/1078-0432.CCR-15-0994. Clin Cancer Res. 2015. PMID: 26240293 No abstract available.
References
-
- Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. - PubMed
-
- Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. - PubMed
-
- Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3:551–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R55 CA109274/CA/NCI NIH HHS/United States
- T32 GM007309/GM/NIGMS NIH HHS/United States
- T32 CA009071/CA/NCI NIH HHS/United States
- CA168180/CA/NCI NIH HHS/United States
- NIH CA088843/CA/NCI NIH HHS/United States
- F31 CA168180/CA/NCI NIH HHS/United States
- CA167939/CA/NCI NIH HHS/United States
- P50 CA088843/CA/NCI NIH HHS/United States
- R01 CA109274/CA/NCI NIH HHS/United States
- GM007309/GM/NIGMS NIH HHS/United States
- CA009071/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- CA109274/CA/NCI NIH HHS/United States
- F31 CA167939/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

