Single continuous lumen formation in the zebrafish gut is mediated by smoothened-dependent tissue remodeling
- PMID: 24504339
- PMCID: PMC3929411
- DOI: 10.1242/dev.100313
Single continuous lumen formation in the zebrafish gut is mediated by smoothened-dependent tissue remodeling
Abstract
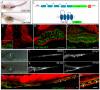
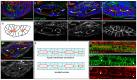
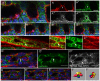
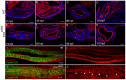
The formation of a single lumen during tubulogenesis is crucial for the development and function of many organs. Although 3D cell culture models have identified molecular mechanisms controlling lumen formation in vitro, their function during vertebrate organogenesis is poorly understood. Using light sheet microscopy and genetic approaches we have investigated single lumen formation in the zebrafish gut. Here we show that during gut development multiple lumens open and enlarge to generate a distinct intermediate, which consists of two adjacent unfused lumens separated by basolateral contacts. We observed that these lumens arise independently from each other along the length of the gut and do not share a continuous apical surface. Resolution of this intermediate into a single, continuous lumen requires the remodeling of contacts between adjacent lumens and subsequent lumen fusion. We show that lumen resolution, but not lumen opening, is impaired in smoothened (smo) mutants, indicating that fluid-driven lumen enlargement and resolution are two distinct processes. Furthermore, we show that smo mutants exhibit perturbations in the Rab11 trafficking pathway and demonstrate that Rab11-mediated trafficking is necessary for single lumen formation. Thus, lumen resolution is a distinct genetically controlled process crucial for single, continuous lumen formation in the zebrafish gut.
Keywords: Lumen; Remodeling; Tubulogenesis.
Figures

References
-
- Bagnat M., Cheung I. D., Mostov K. E., Stainier D. Y. R. (2007). Genetic control of single lumen formation in the zebrafish gut. Nat. Cell Biol. 9, 954–960 - PubMed
-
- Chen W., Burgess S., Hopkins N. (2001). Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development 128, 2385–2396 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

