Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases
- PMID: 24504409
- PMCID: PMC4011661
- DOI: 10.1038/nm.3457
Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases
Abstract
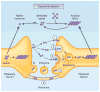
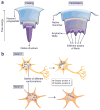
A common feature of many neurodegenerative diseases is the deposition of β-sheet-rich amyloid aggregates formed by proteins specific to these diseases. These protein aggregates are thought to cause neuronal dysfunction, directly or indirectly. Recent studies have strongly implicated cell-to-cell transmission of misfolded proteins as a common mechanism for the onset and progression of various neurodegenerative disorders. Emerging evidence also suggests the presence of conformationally diverse 'strains' of each type of disease protein, which may be another shared feature of amyloid aggregates, accounting for the tremendous heterogeneity within each type of neurodegenerative disease. Although there are many more questions to be answered, these studies have opened up new avenues for therapeutic interventions in neurodegenerative disorders.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
-
- DiFiglia M, et al. Aggregation of hunting tin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

