Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition
- PMID: 24504412
- PMCID: PMC3974457
- DOI: 10.1242/dmm.014126
Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition
Abstract
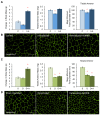
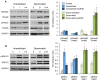
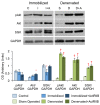
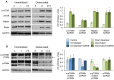
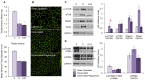
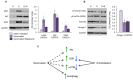
The purpose of our study was to compare two acquired muscle atrophies and the use of myostatin inhibition for their treatment. Myostatin naturally inhibits skeletal muscle growth by binding to ActRIIB, a receptor on the cell surface of myofibers. Because blocking myostatin in an adult wild-type mouse induces profound muscle hypertrophy, we applied a soluble ActRIIB receptor to models of disuse (limb immobilization) and denervation (sciatic nerve resection) atrophy. We found that treatment of immobilized mice with ActRIIB prevented the loss of muscle mass observed in placebo-treated mice. Our results suggest that this protection from disuse atrophy is regulated by serum and glucocorticoid-induced kinase (SGK) rather than by Akt. Denervation atrophy, however, was not protected by ActRIIB treatment, yet resulted in an upregulation of the pro-growth factors Akt, SGK and components of the mTOR pathway. We then treated the denervated mice with the mTOR inhibitor rapamycin and found that, despite a reduction in mTOR activation, there is no alteration of the atrophy phenotype. Additionally, rapamycin prevented the denervation-induced upregulation of the mTORC2 substrates Akt and SGK. Thus, our studies show that denervation atrophy is not only independent from Akt, SGK and mTOR activation but also has a different underlying pathophysiological mechanism than disuse atrophy.
Keywords: Denervation atrophy; Muscle atrophy pathophysiology; Myostatin; Skeletal muscle; TGF-β signaling.
Figures

References
-
- Andres-Mateos E., Brinkmeier H., Burks T. N., Mejias R., Files D. C., Steinberger M., Soleimani A., Marx R., Simmers J. L., Lin B., et al. (2013). Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol. Med. 5, 80–91 - PMC - PubMed
-
- Aoyama T., Matsui T., Novikov M., Park J., Hemmings B., Rosenzweig A. (2005). Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation. 111, 1652–1659 - PubMed
-
- Baumann A. P., Ibebunjo C., Grasser W. A., Paralkar V. M. (2003). Myostatin expression in age and denervation-induced skeletal muscle atrophy. J. Musculoskelet. Neuronal Interact. 3, 8–16 - PubMed
-
- Bodine S. C., Latres E., Baumhueter S., Lai V. K., Nunez L., Clarke B. A., Poueymirou W. T., Panaro F. J., Na E., Dharmarajan K., et al. (2001a). Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 294, 1704–1708 - PubMed
-
- Bodine S. C., Stitt T. N., Gonzalez M., Kline W. O., Stover G. L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J. C., Glass D. J., et al. (2001b). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3, 1014–1019 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

